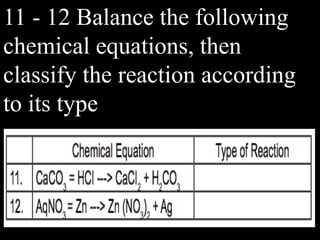
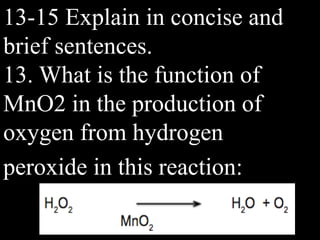
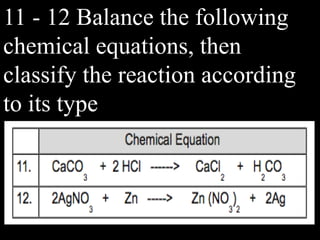
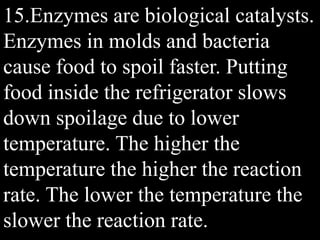
This document provides an overview of key concepts related to chemical reactions, including writing and balancing chemical equations, factors that affect reaction rates such as concentration, temperature, surface area, and catalysts. It also discusses how an understanding of reaction rates can be applied to food preservation, materials production, pollution control, and corrosion prevention. A pre-assessment with multiple choice and true/false questions is included to gauge students' existing knowledge on these topics.