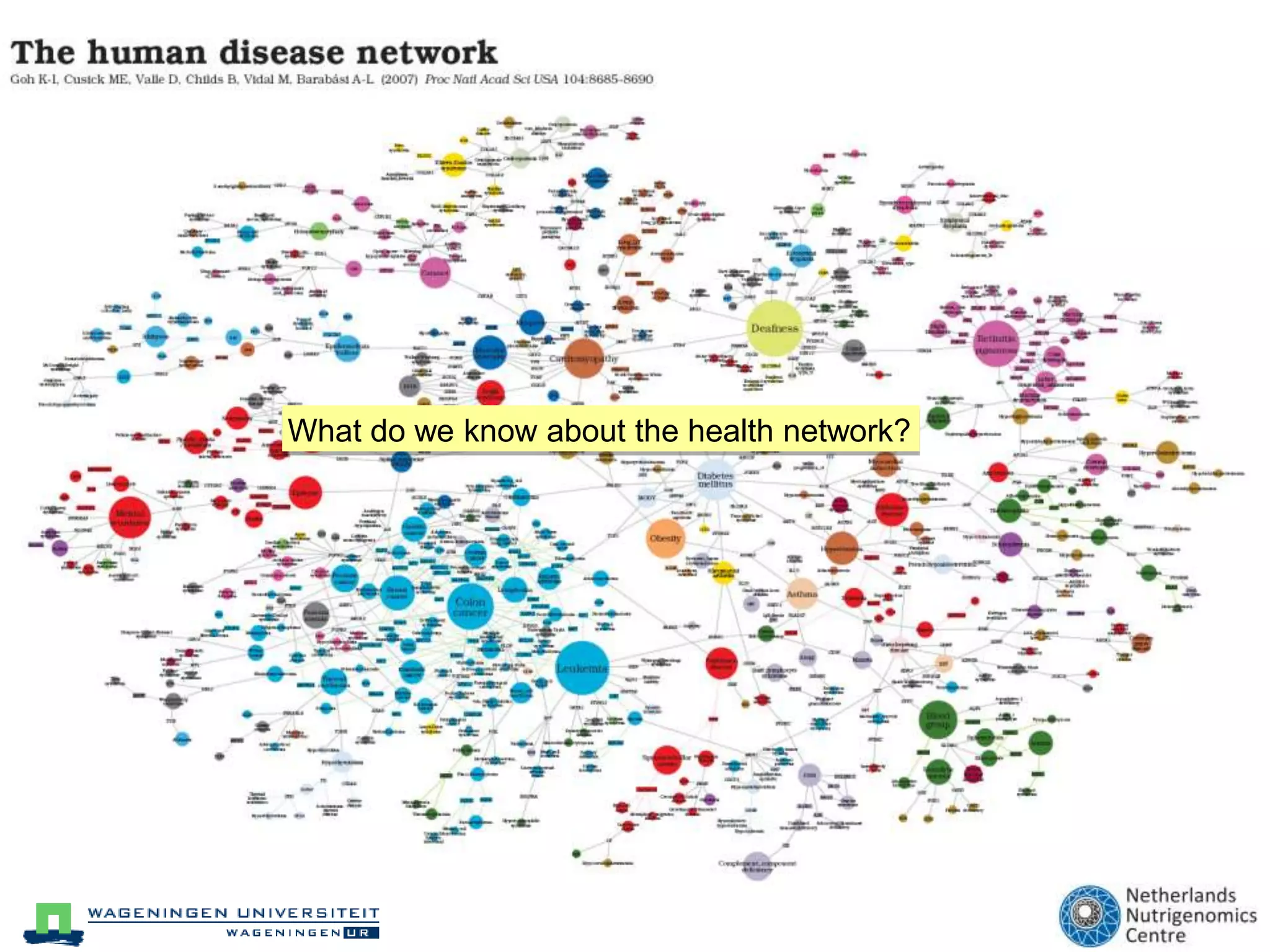
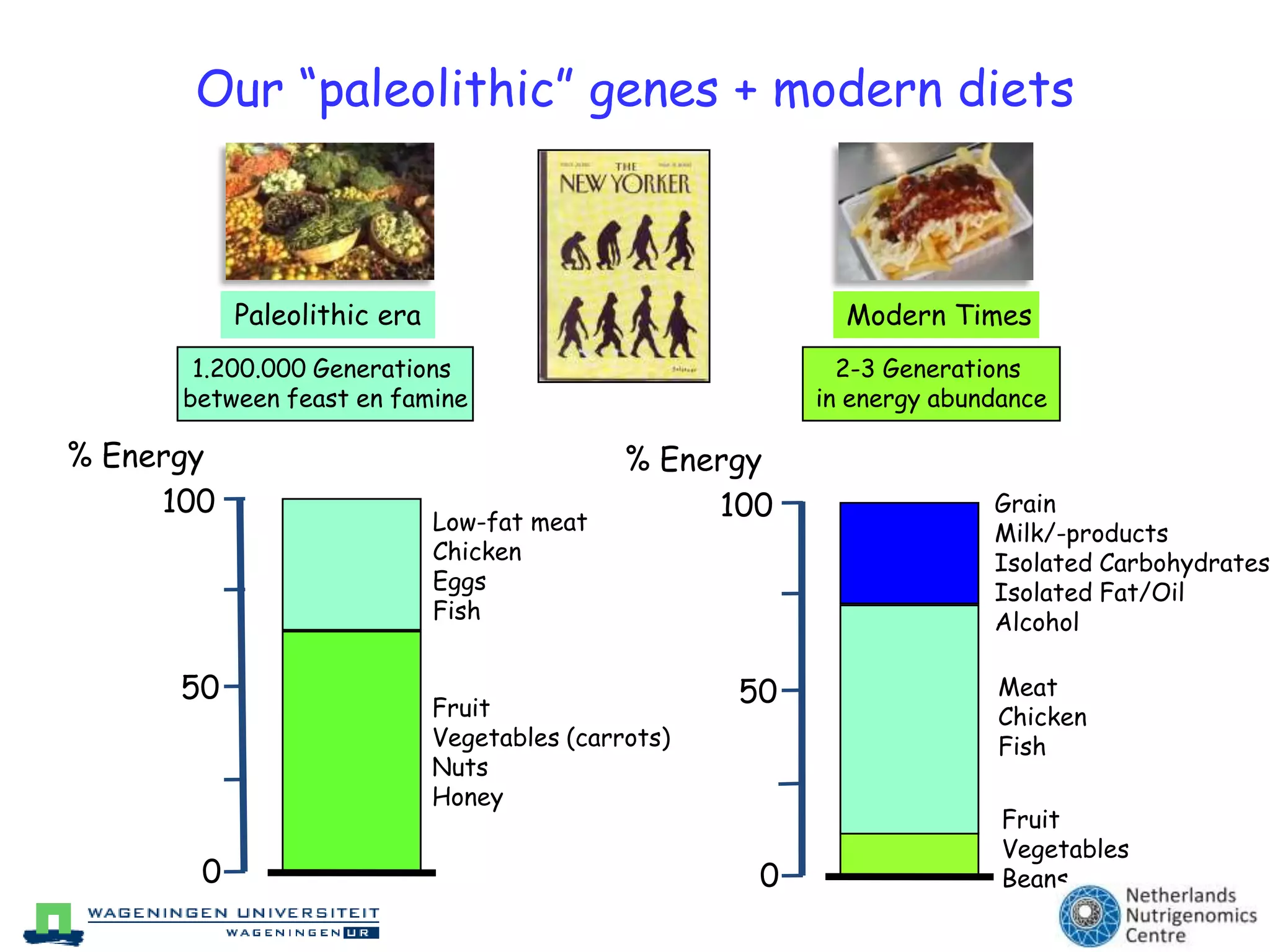
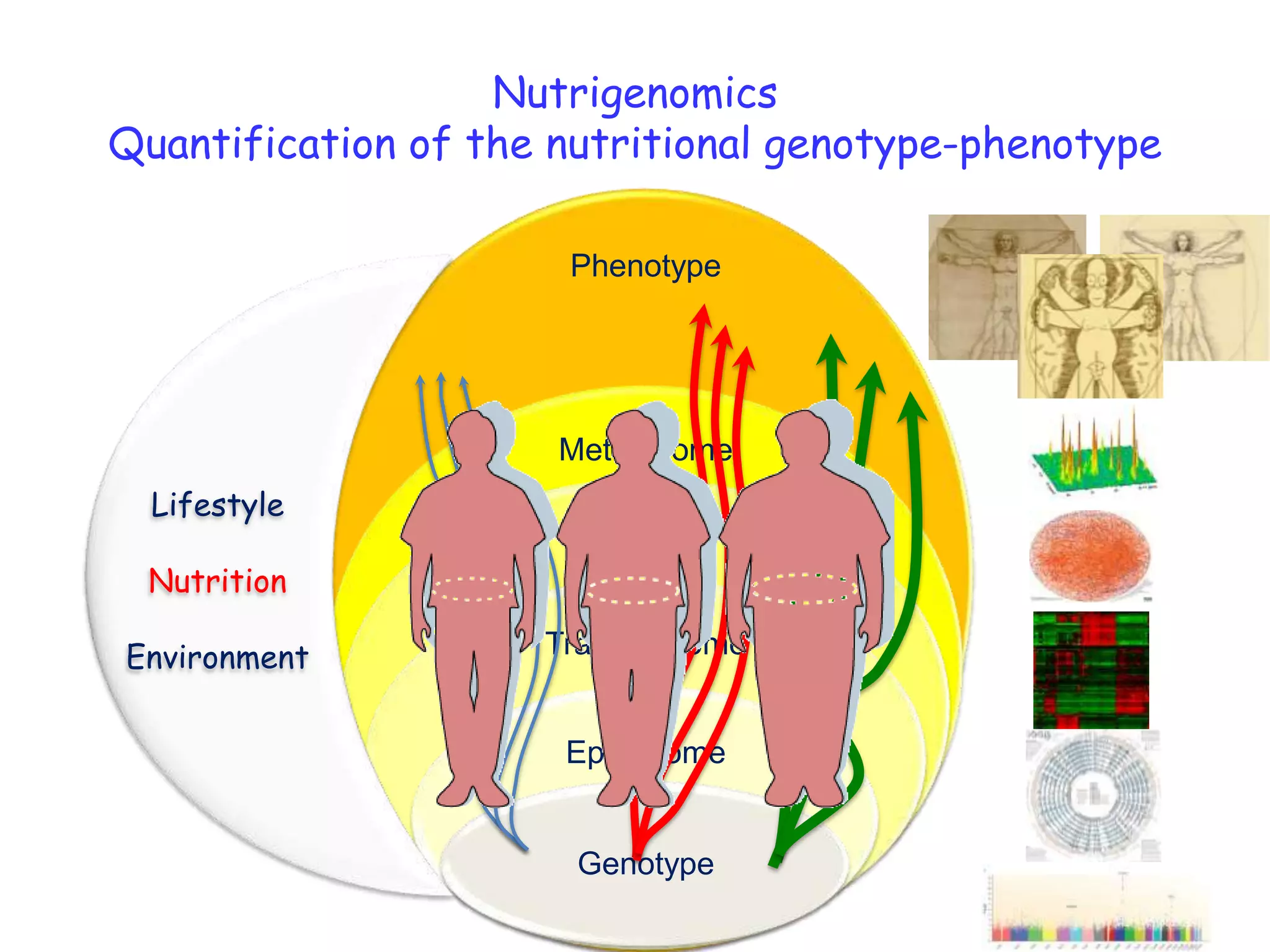
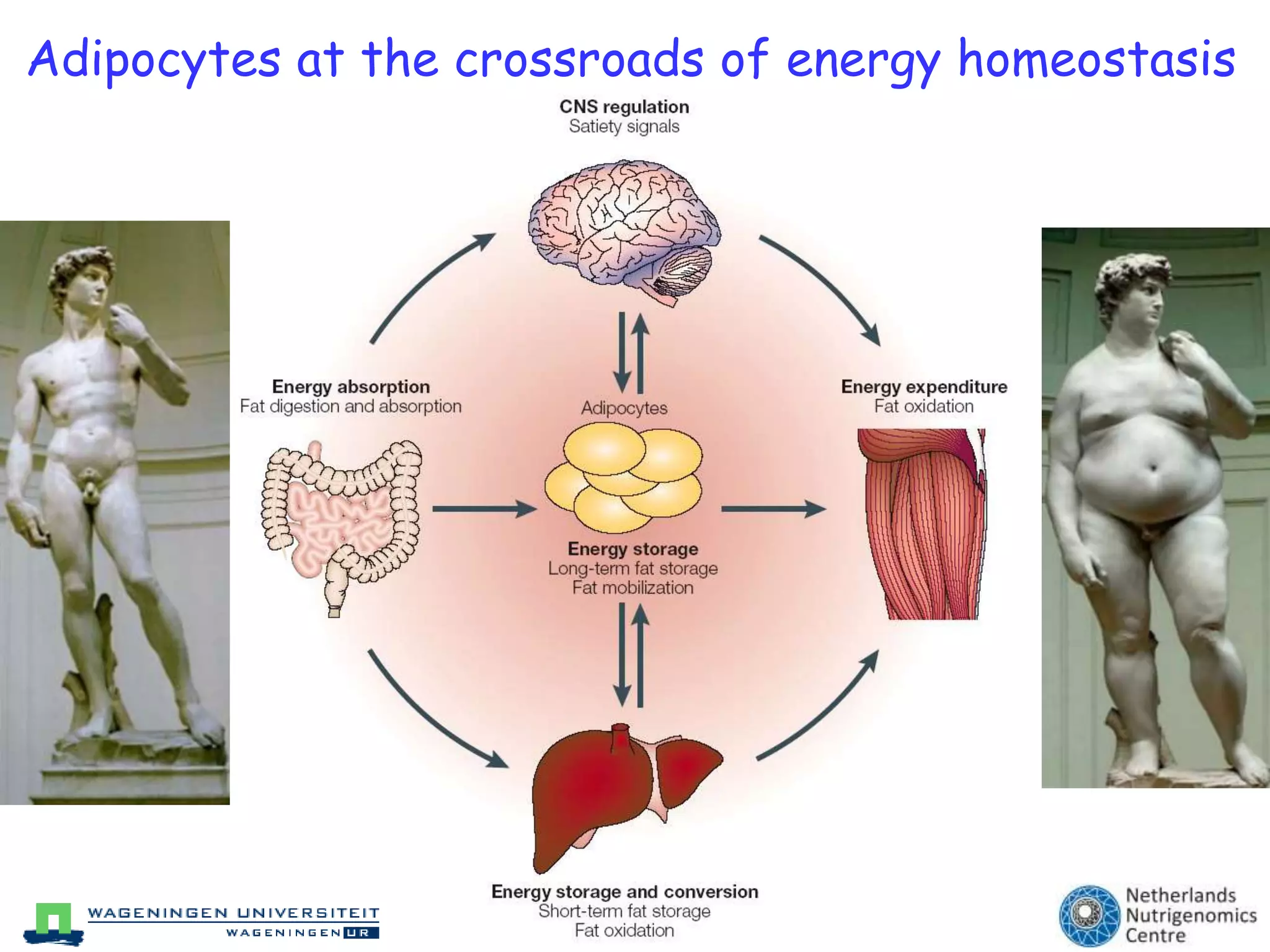
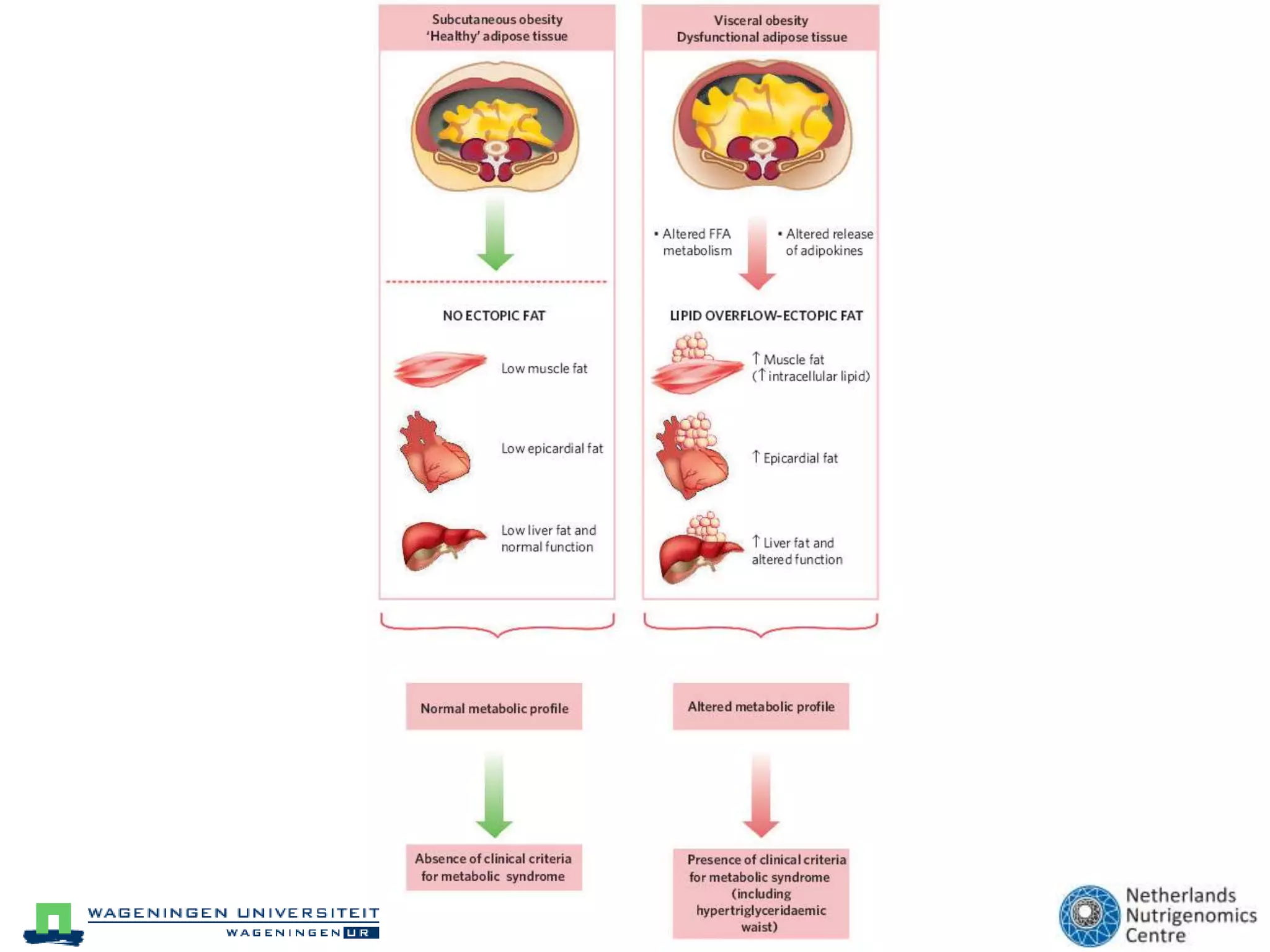
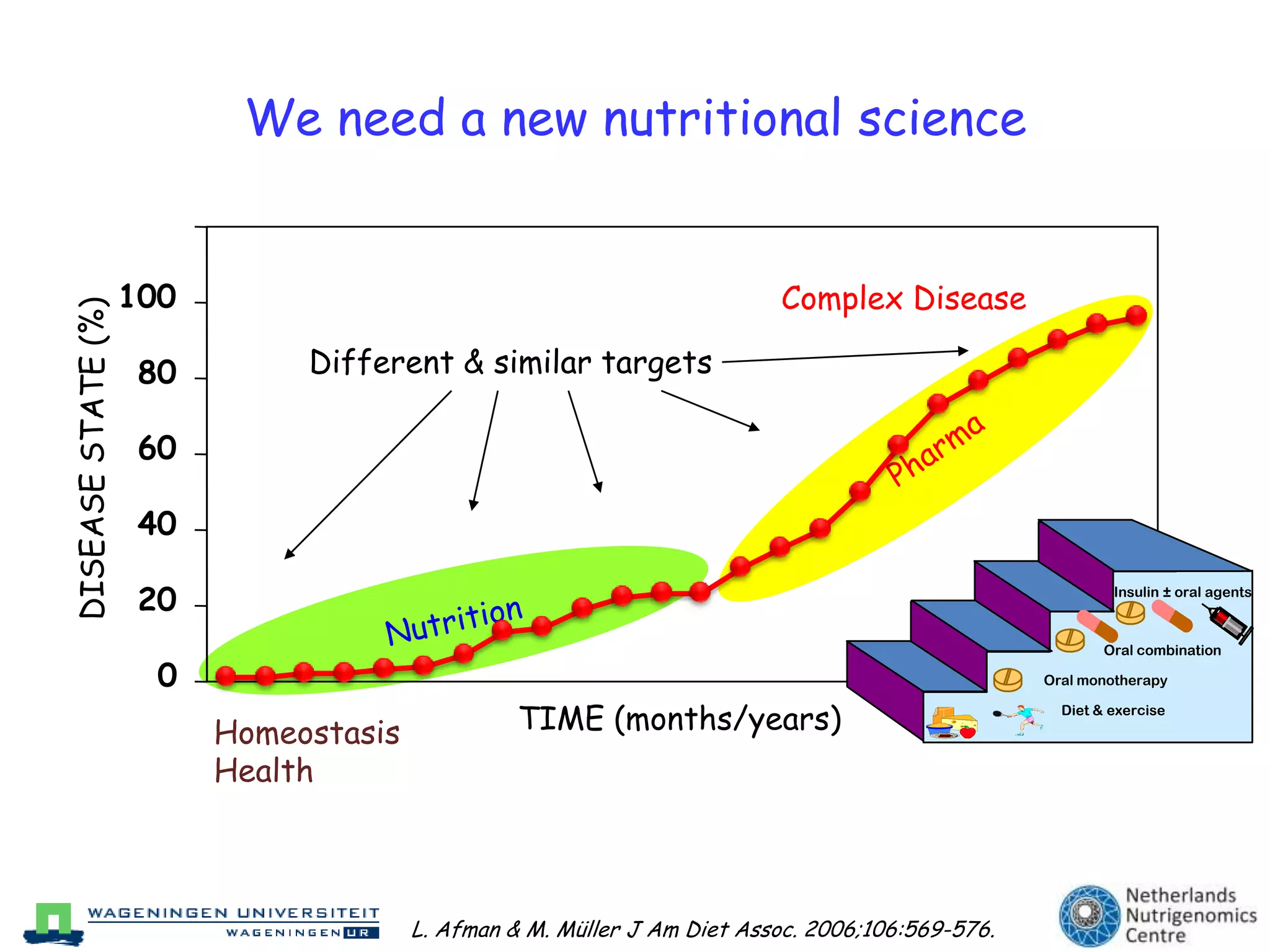
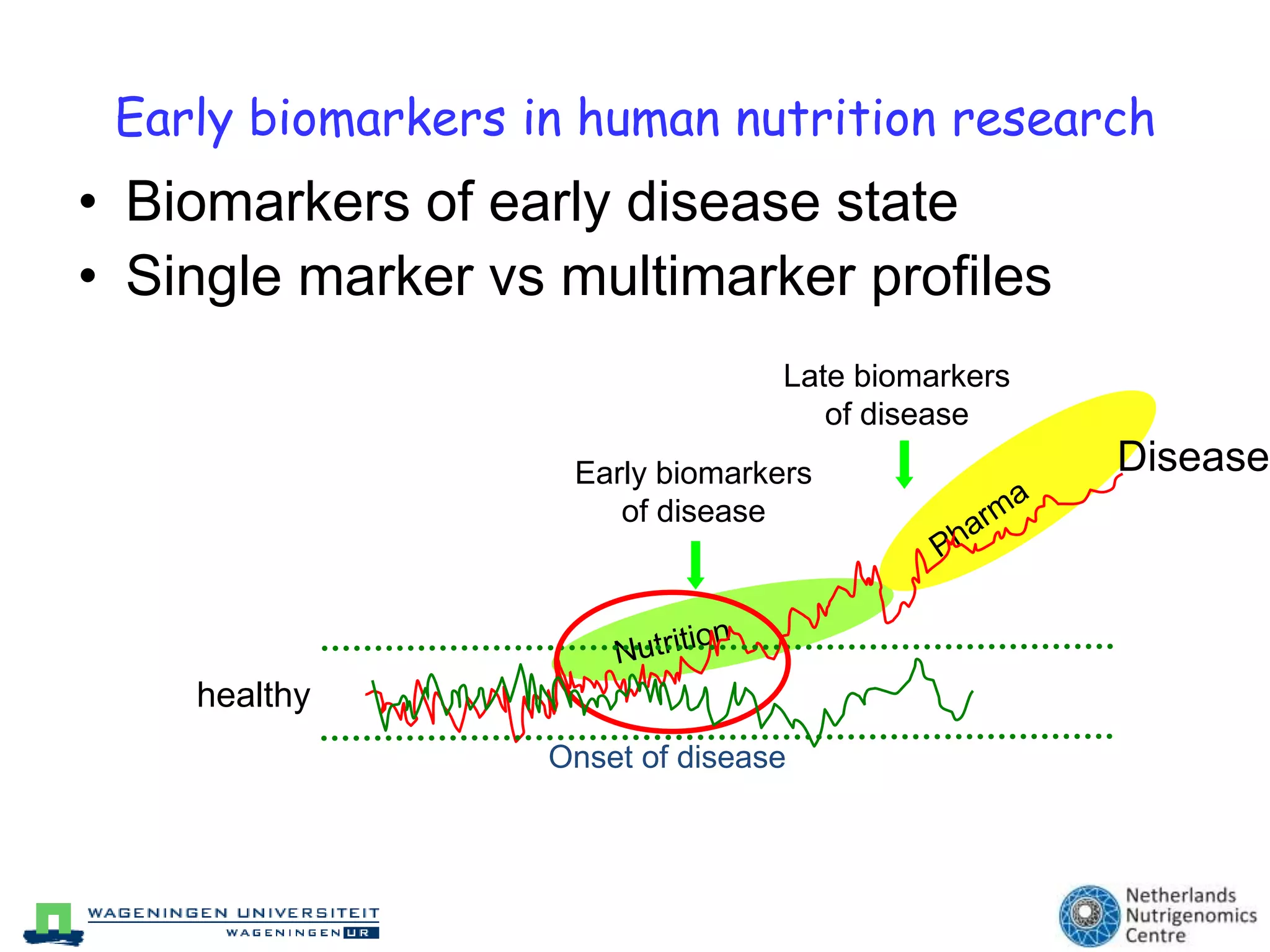
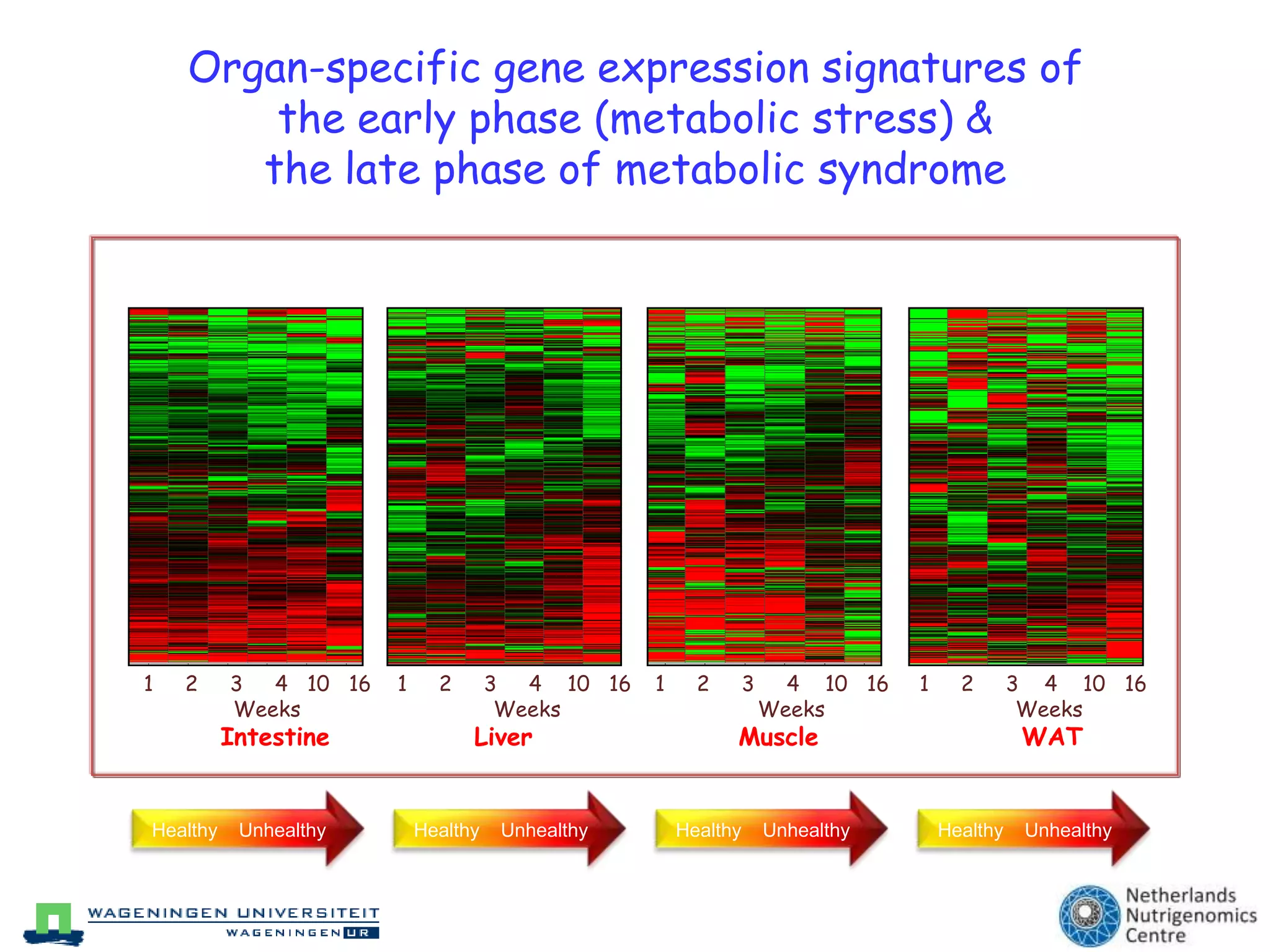
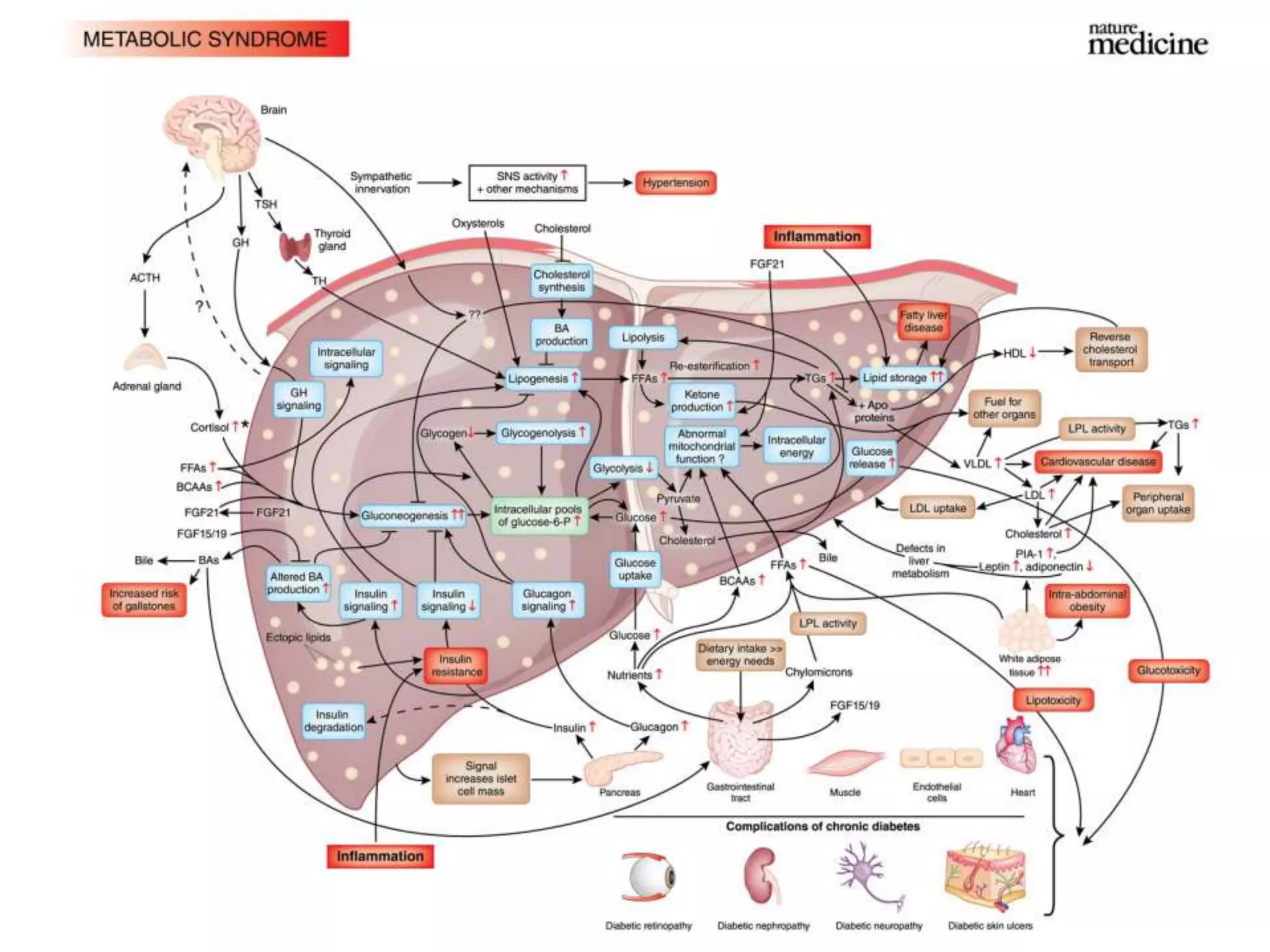
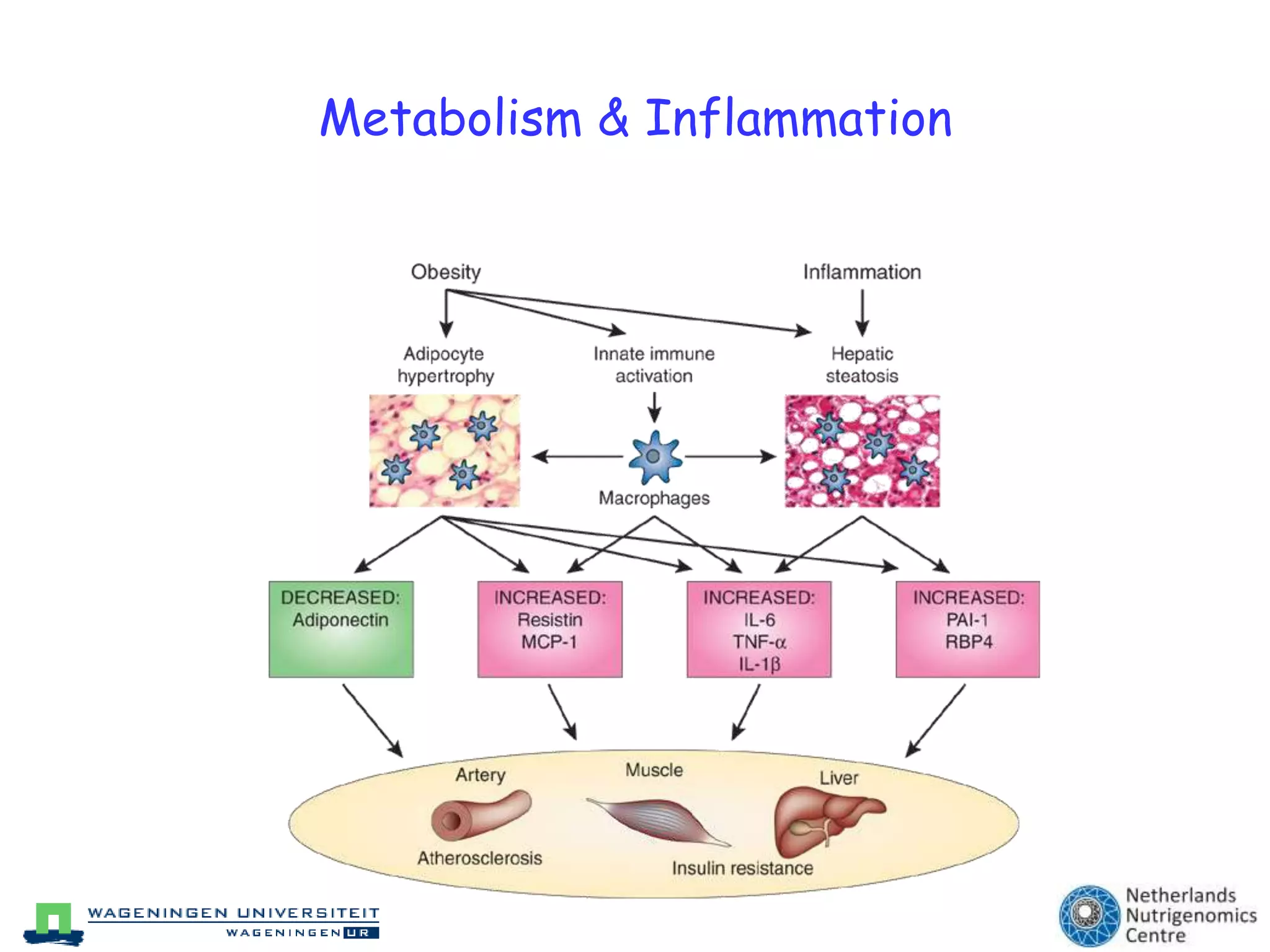
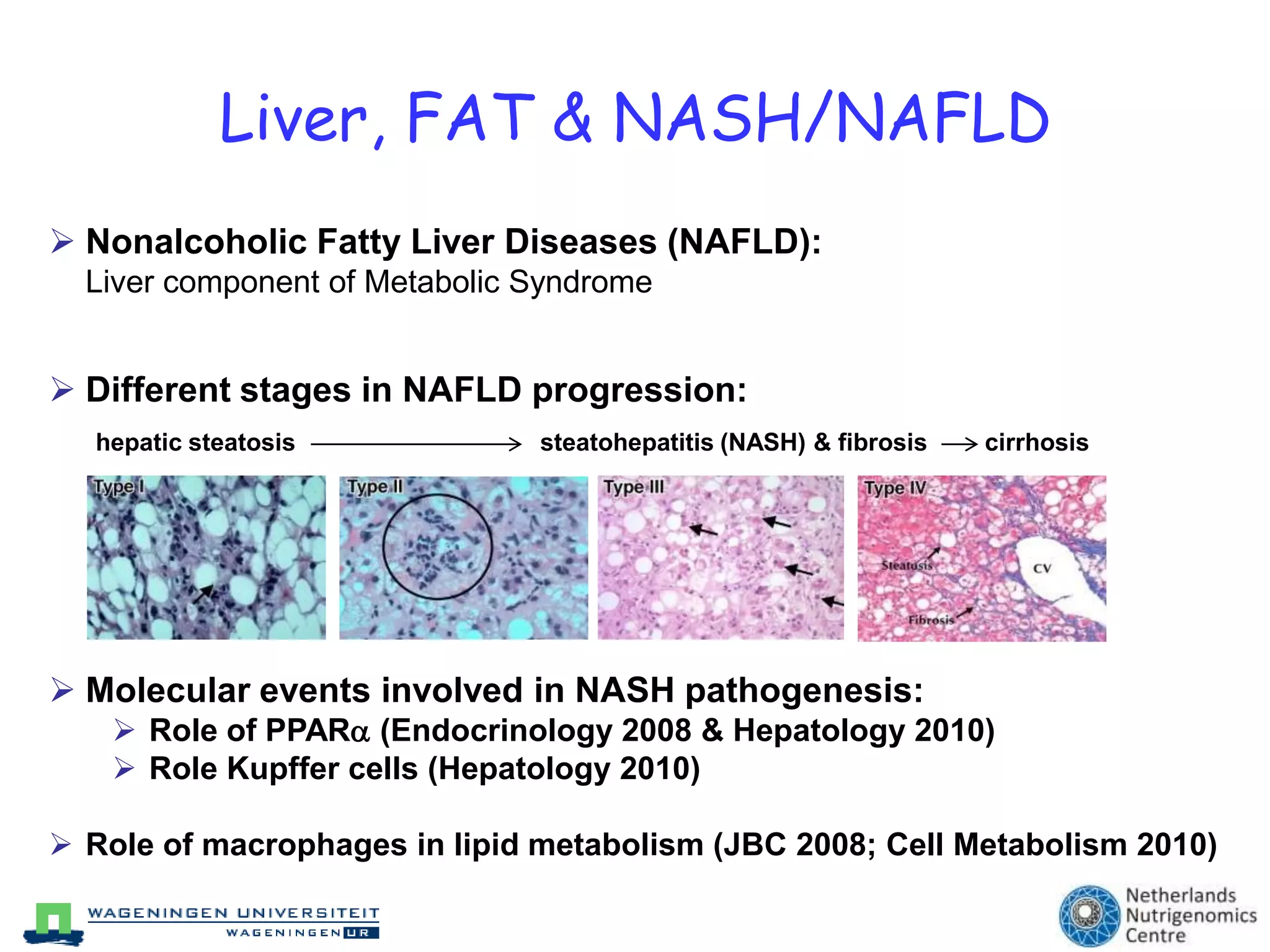
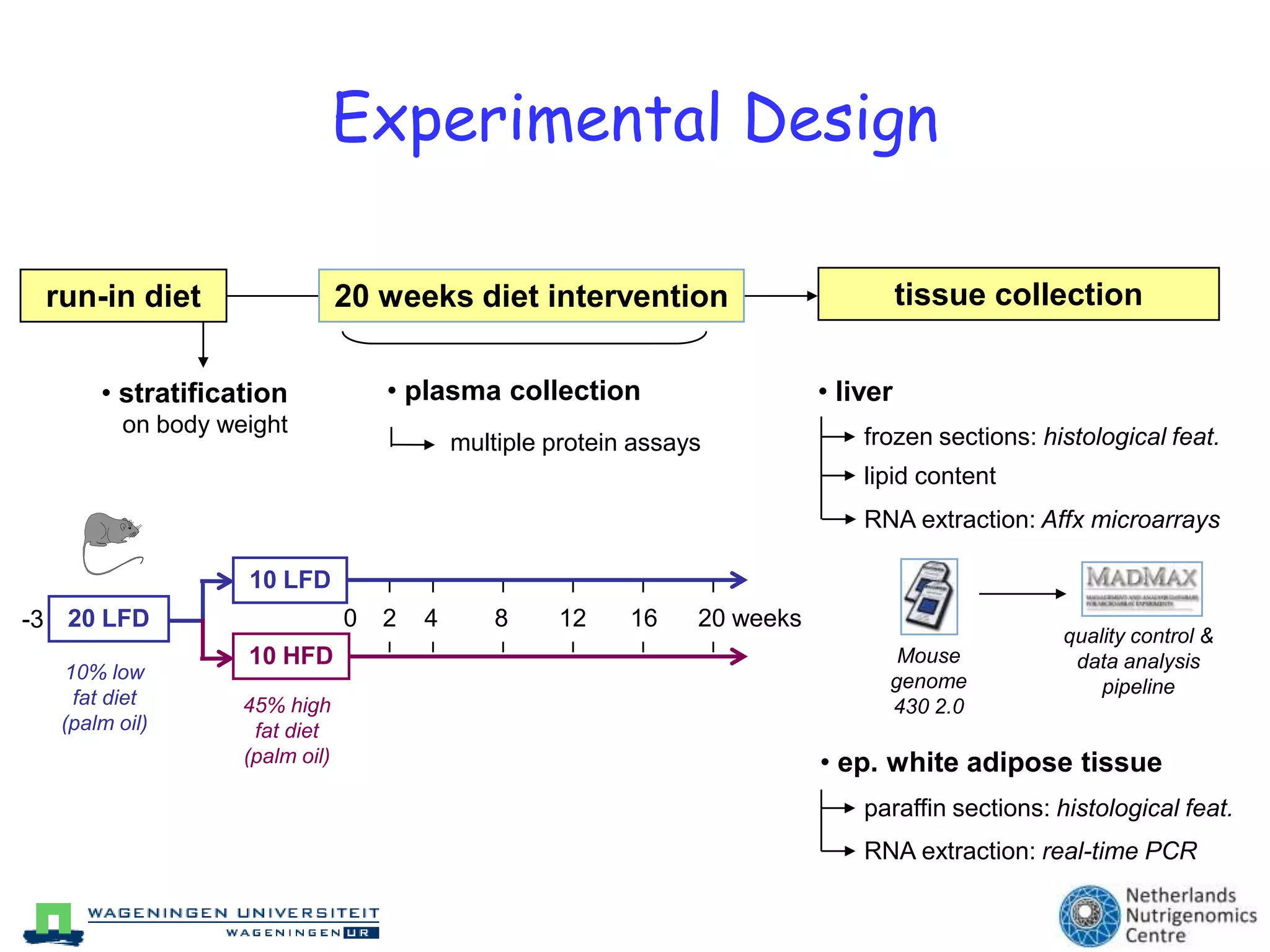
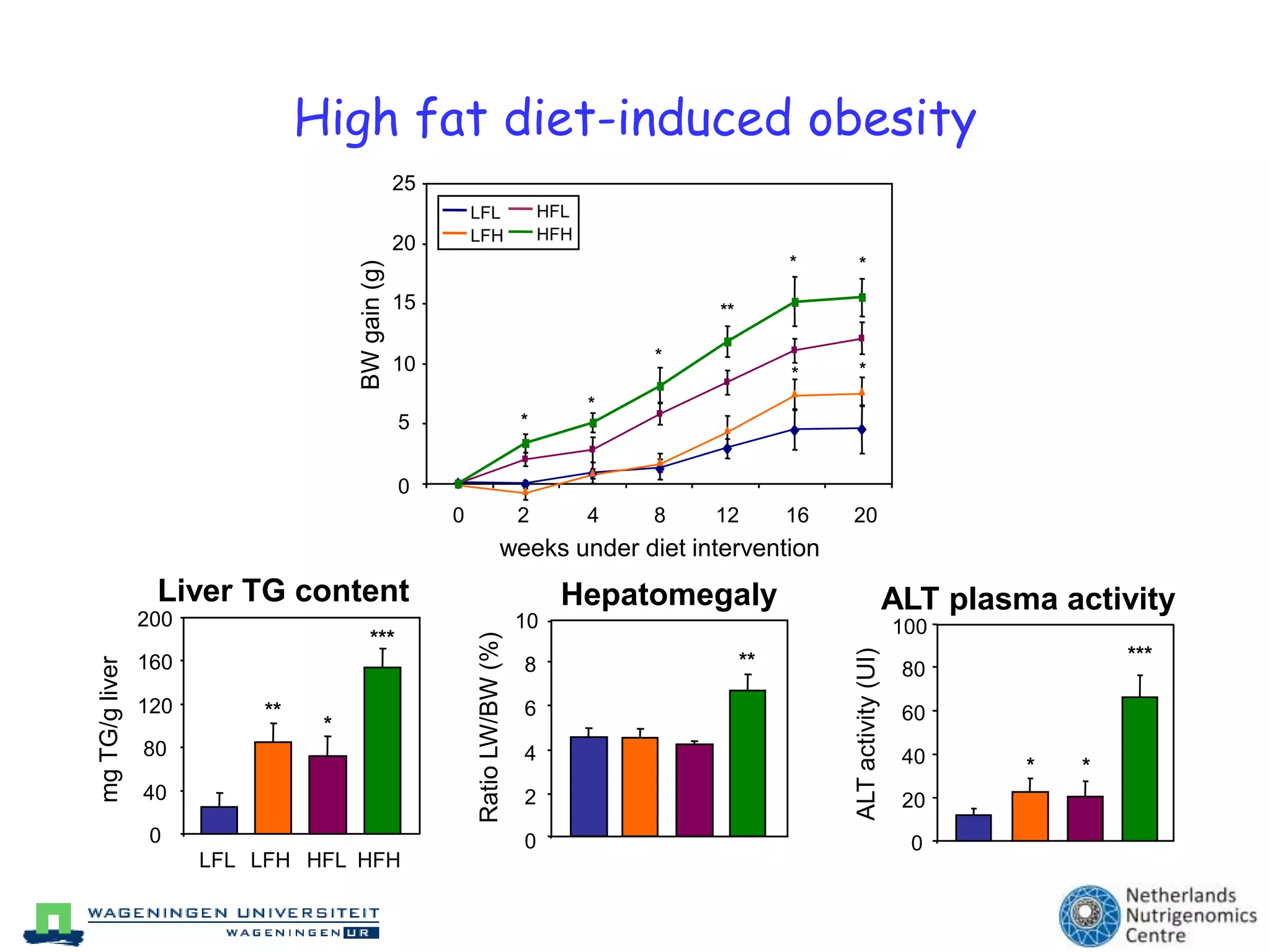
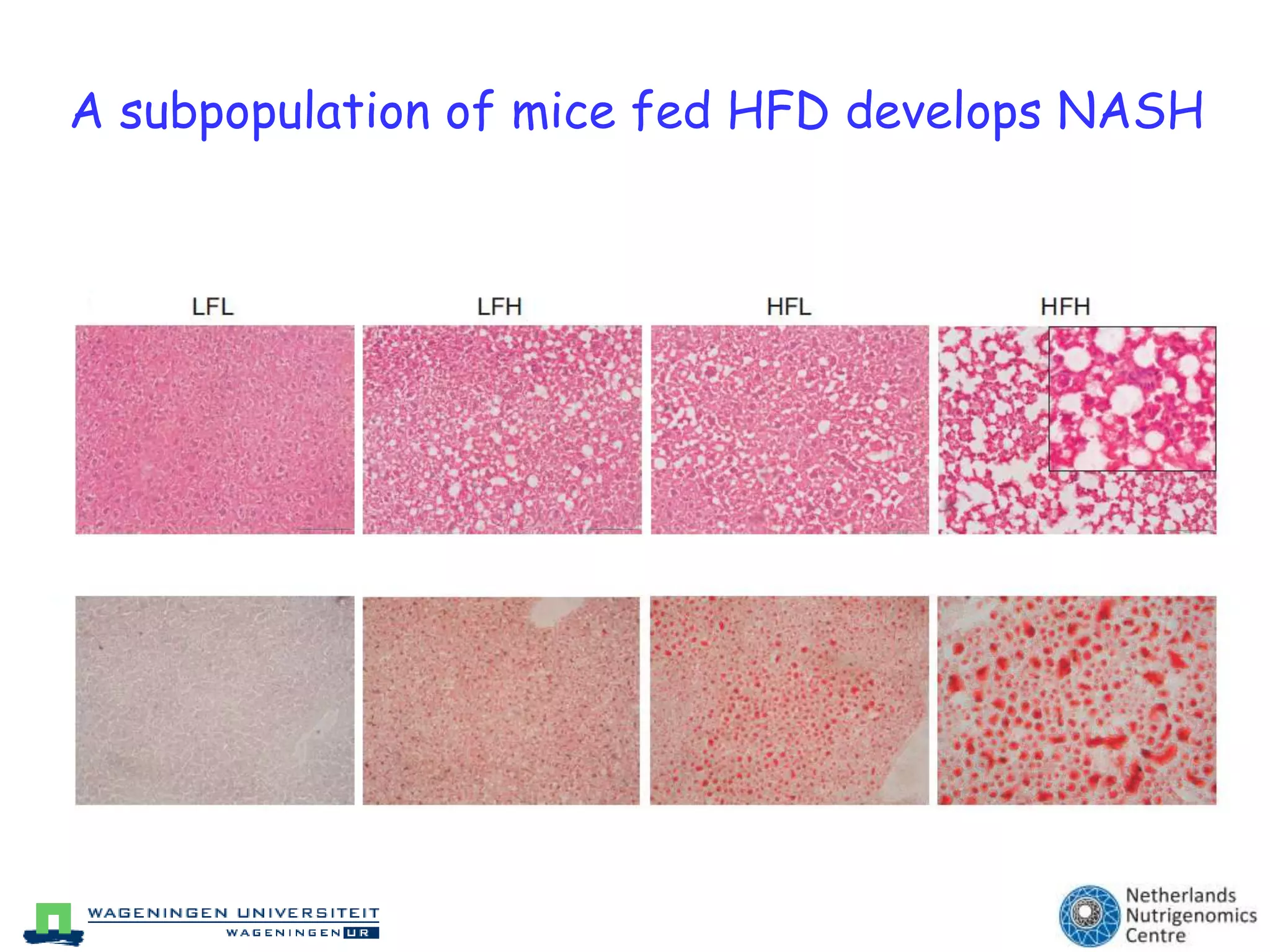
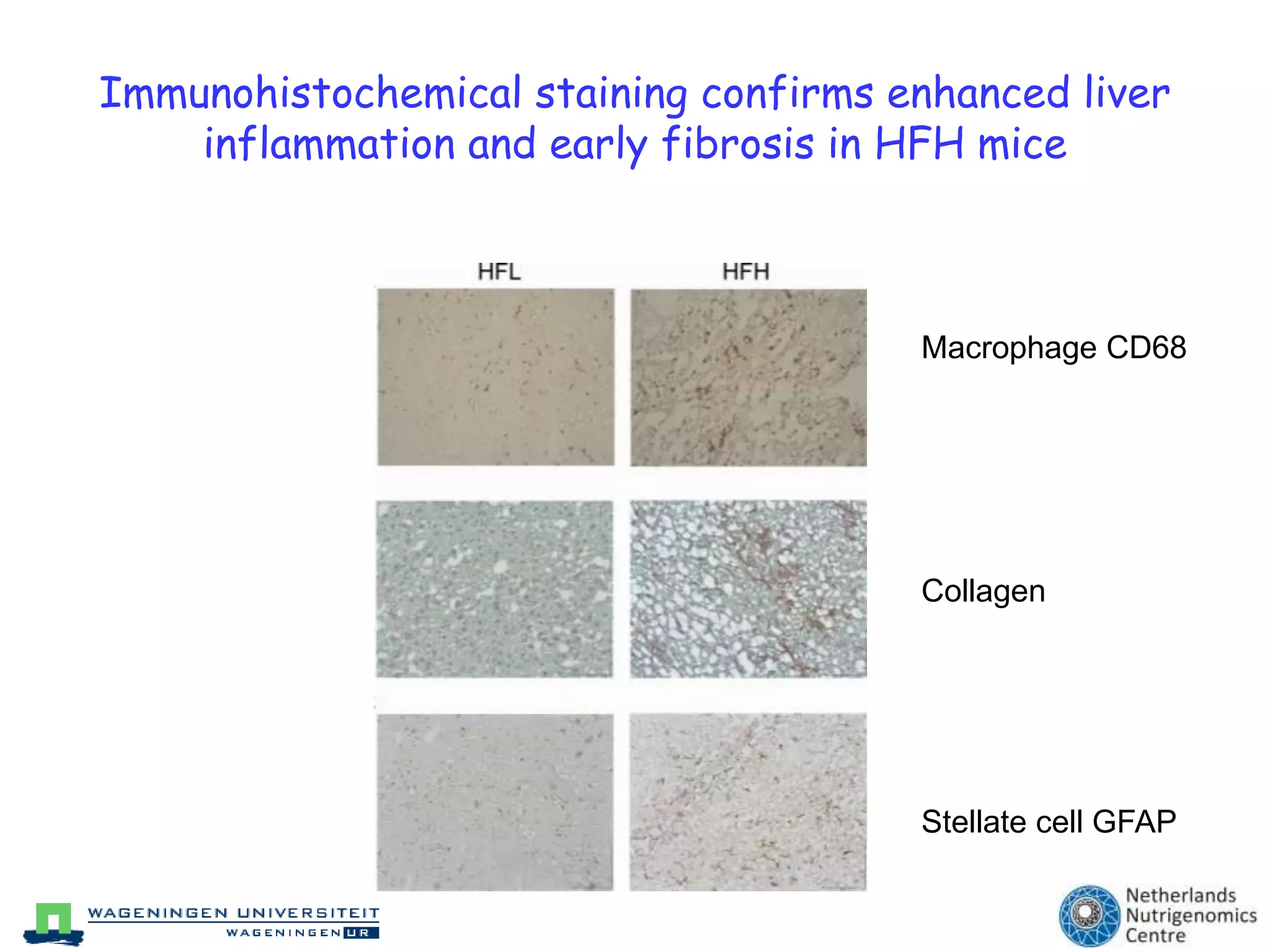
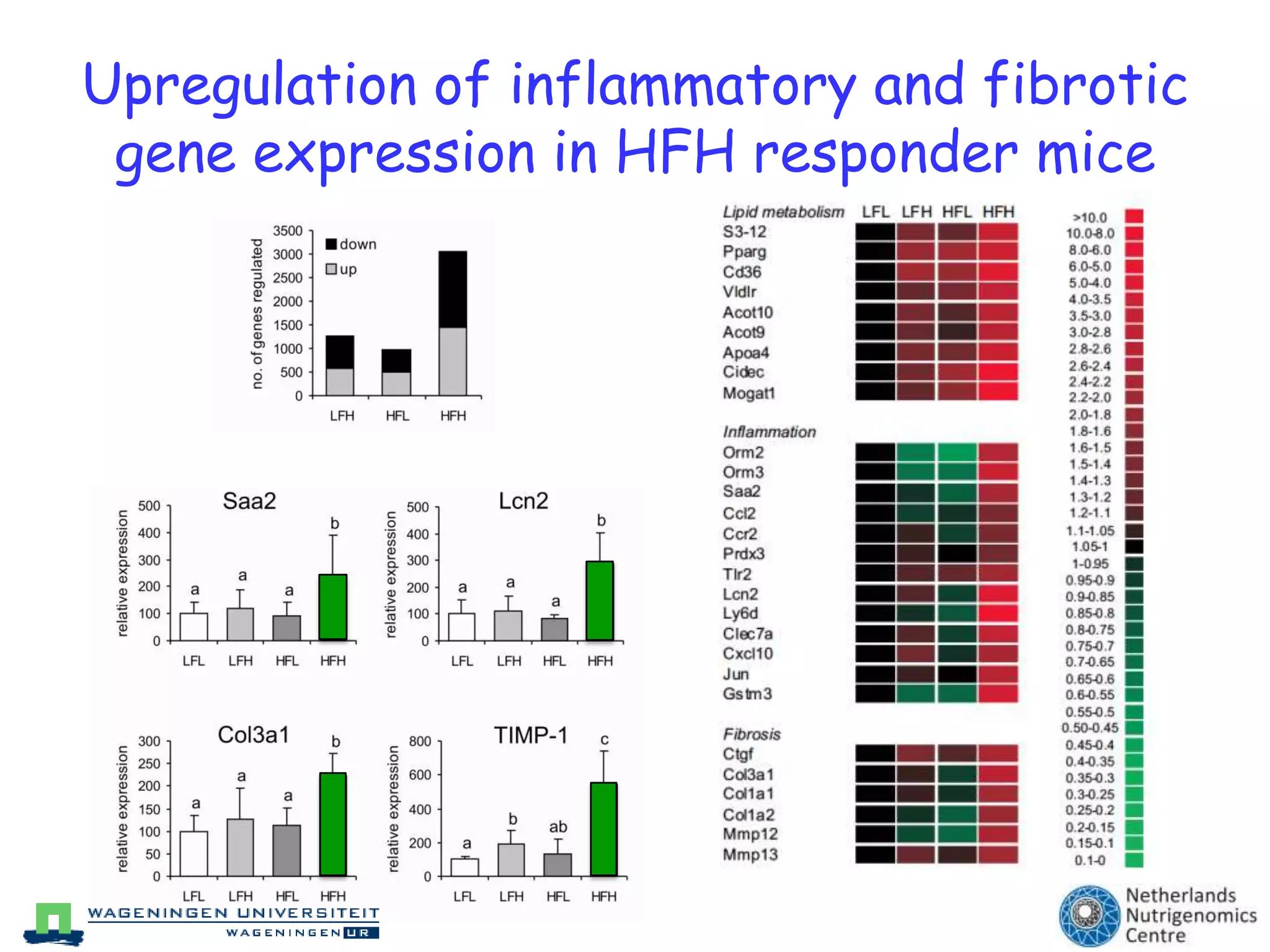
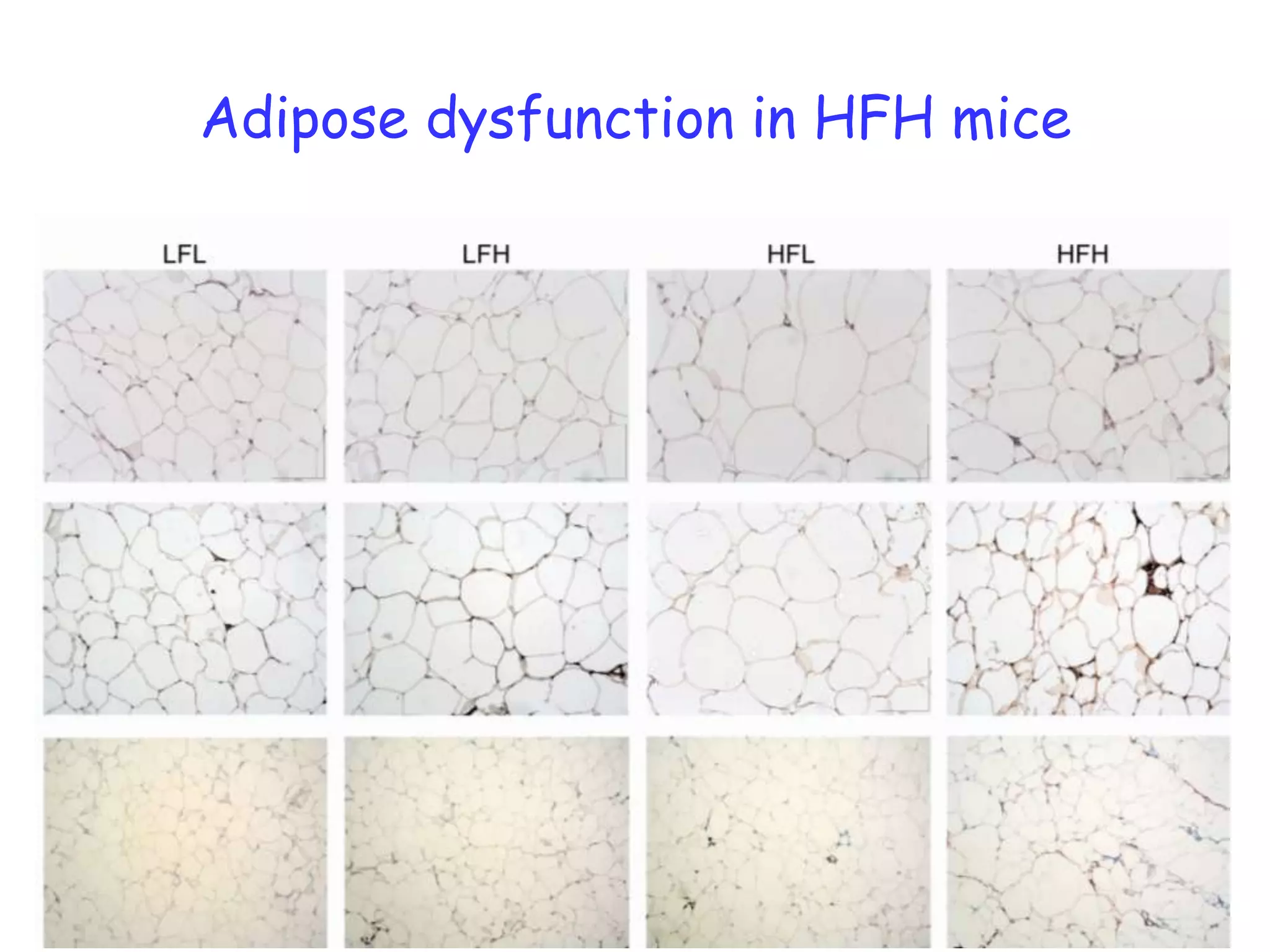
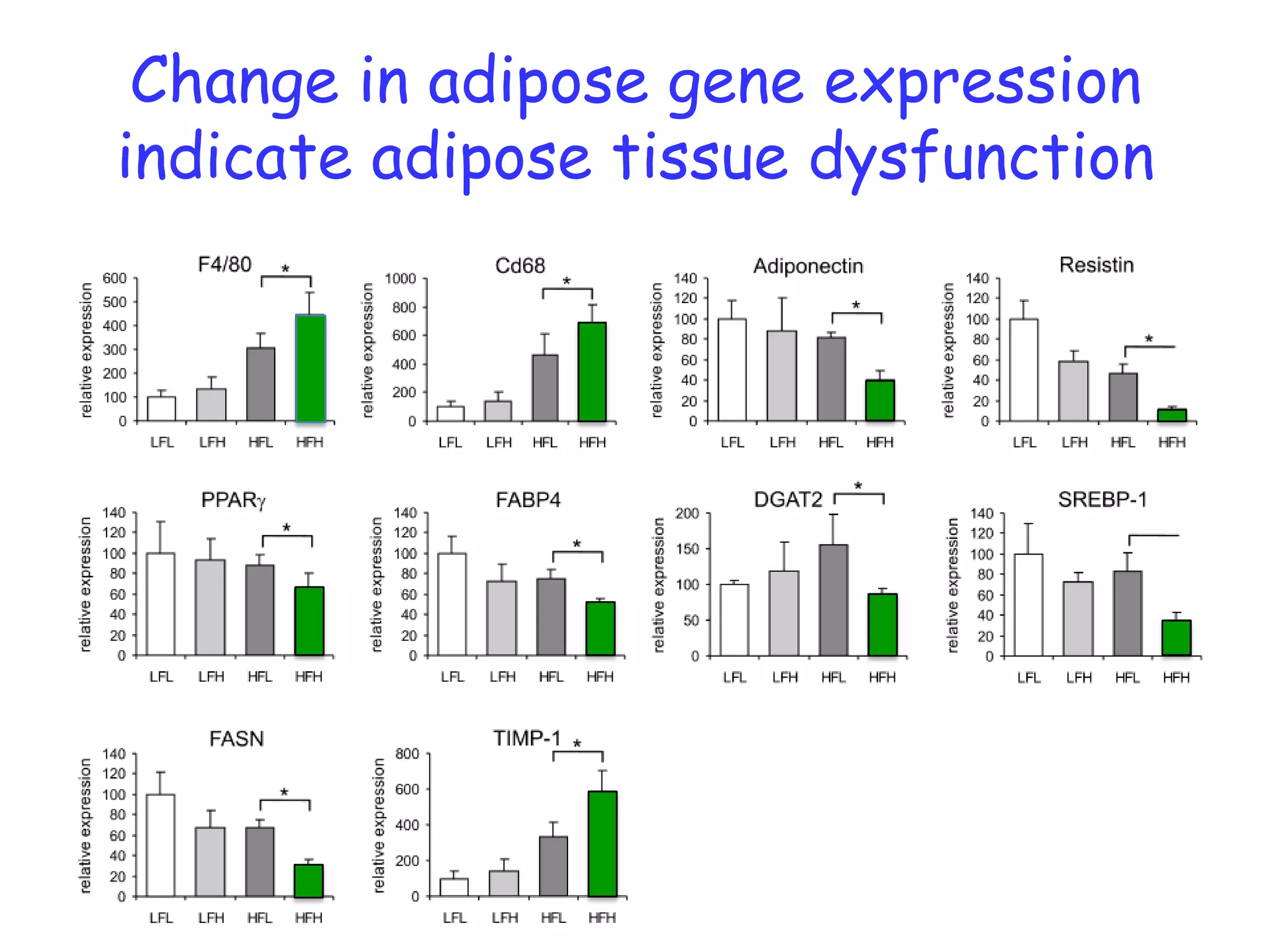
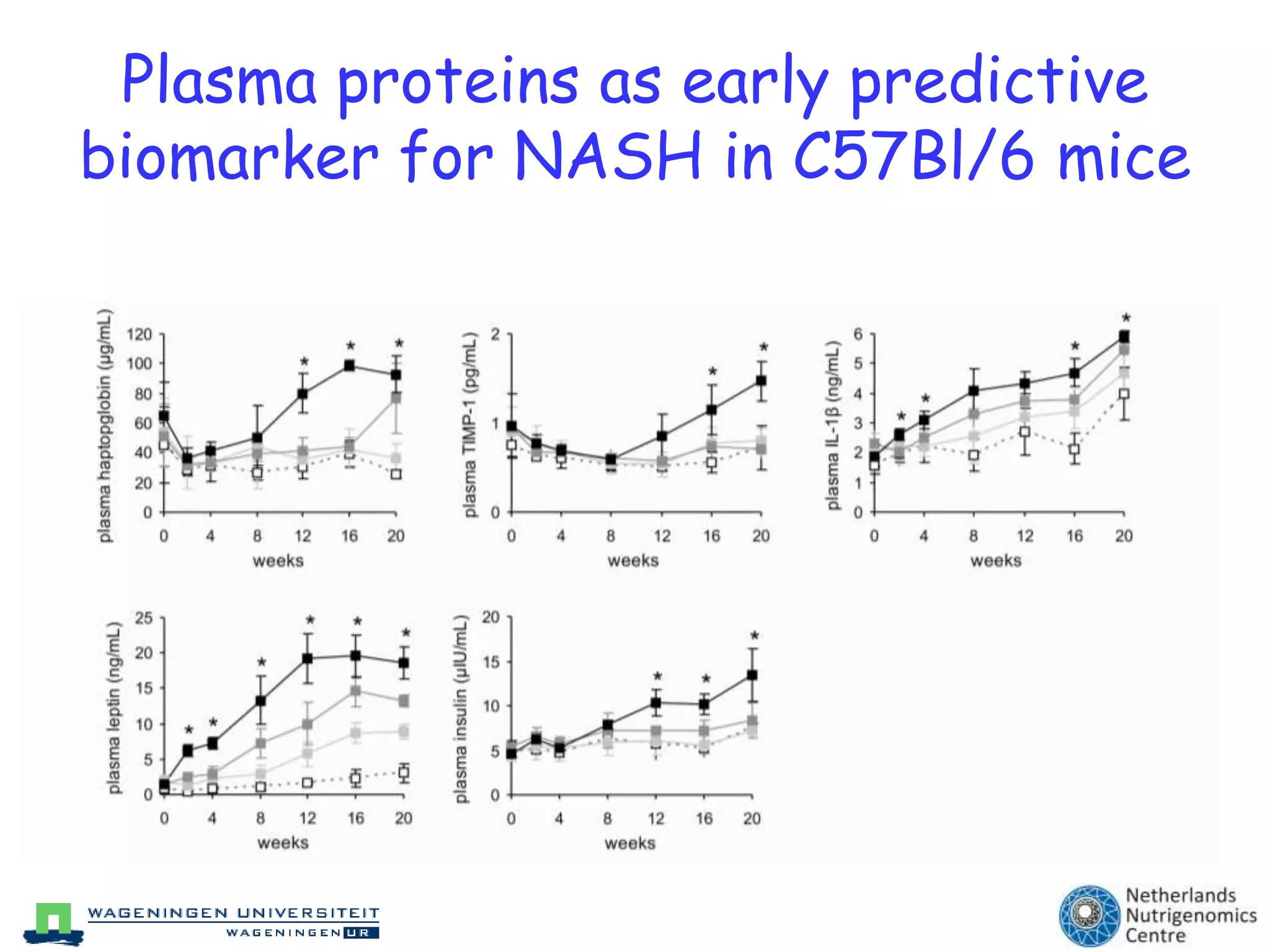
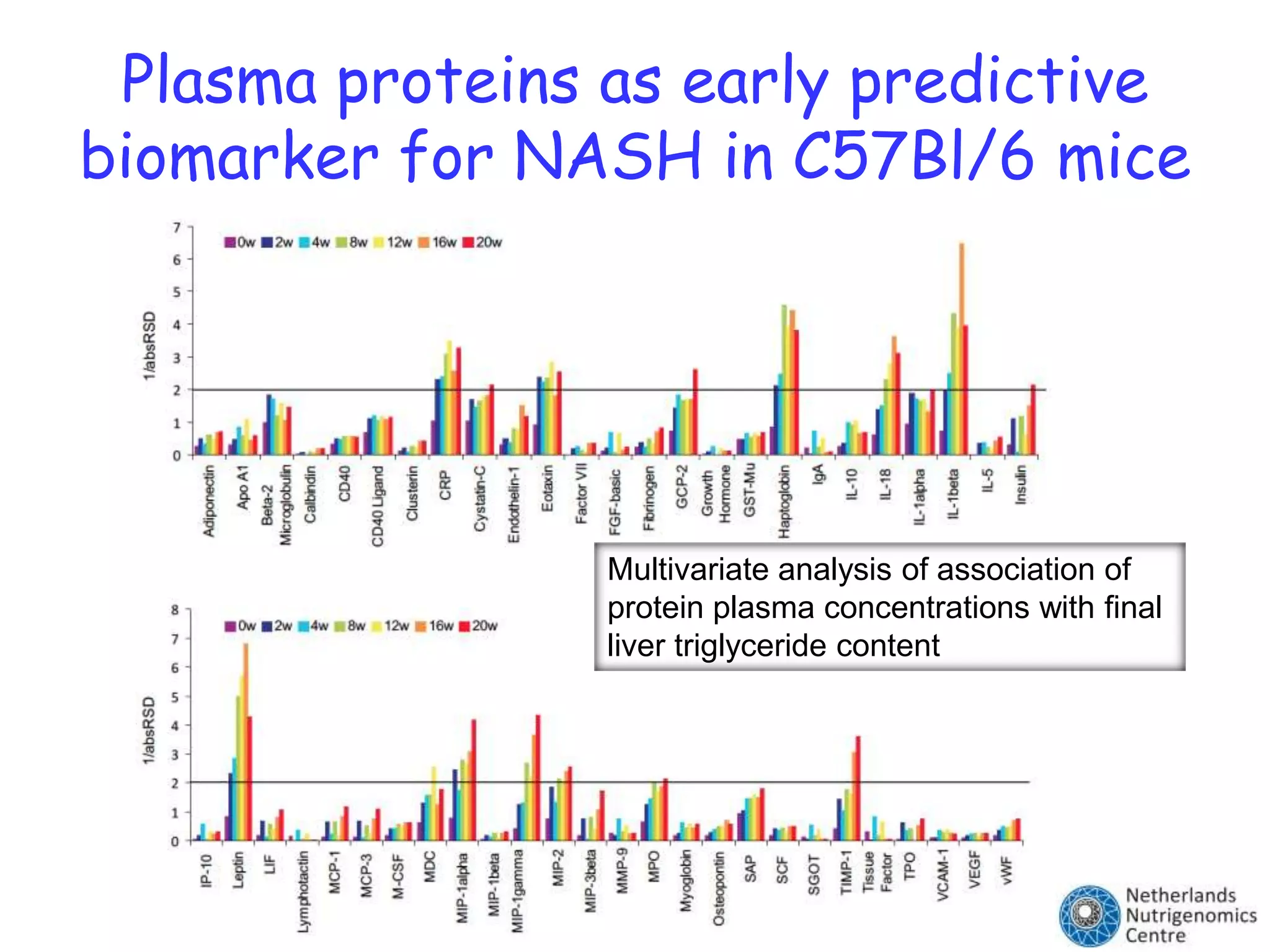
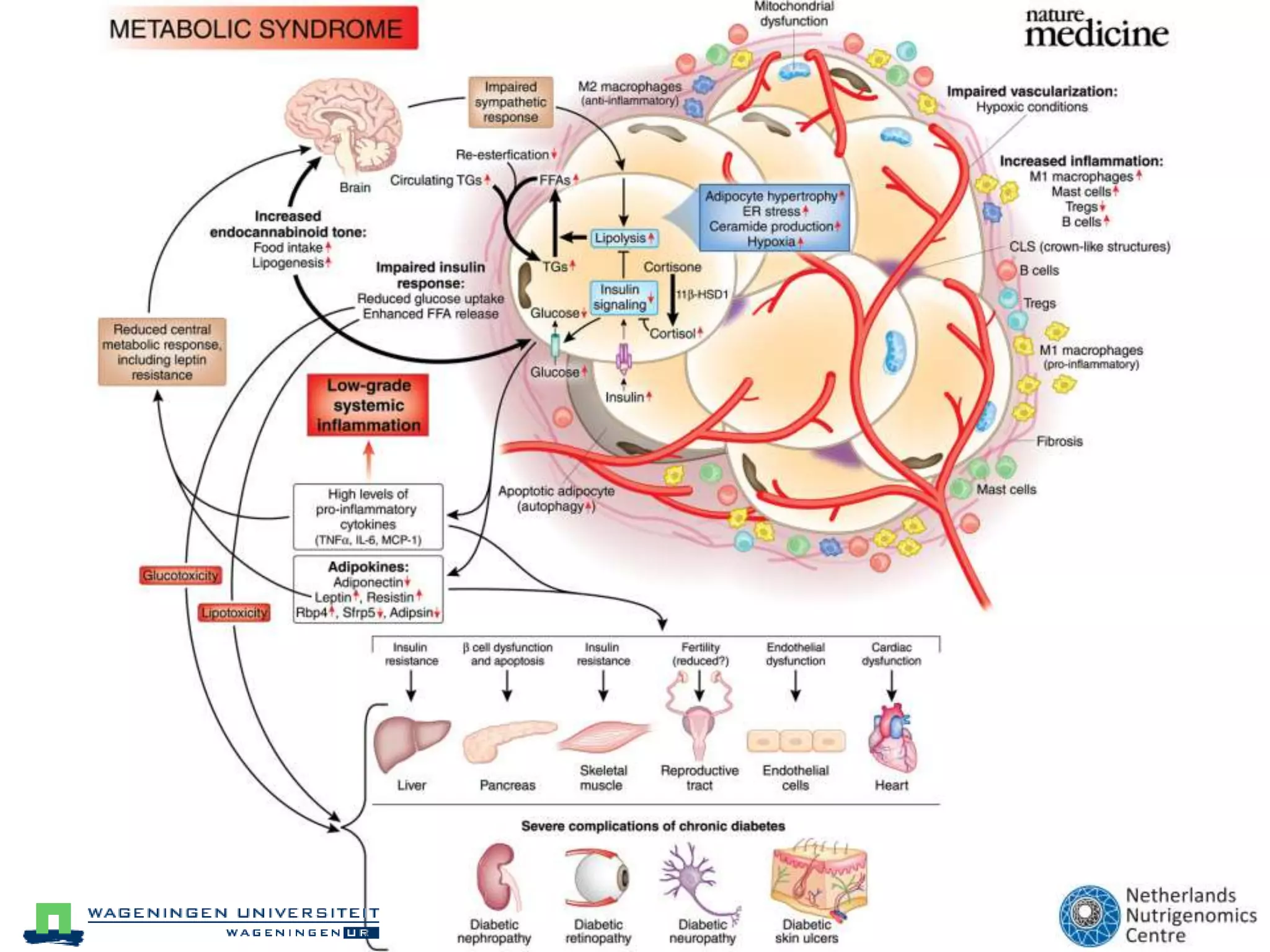
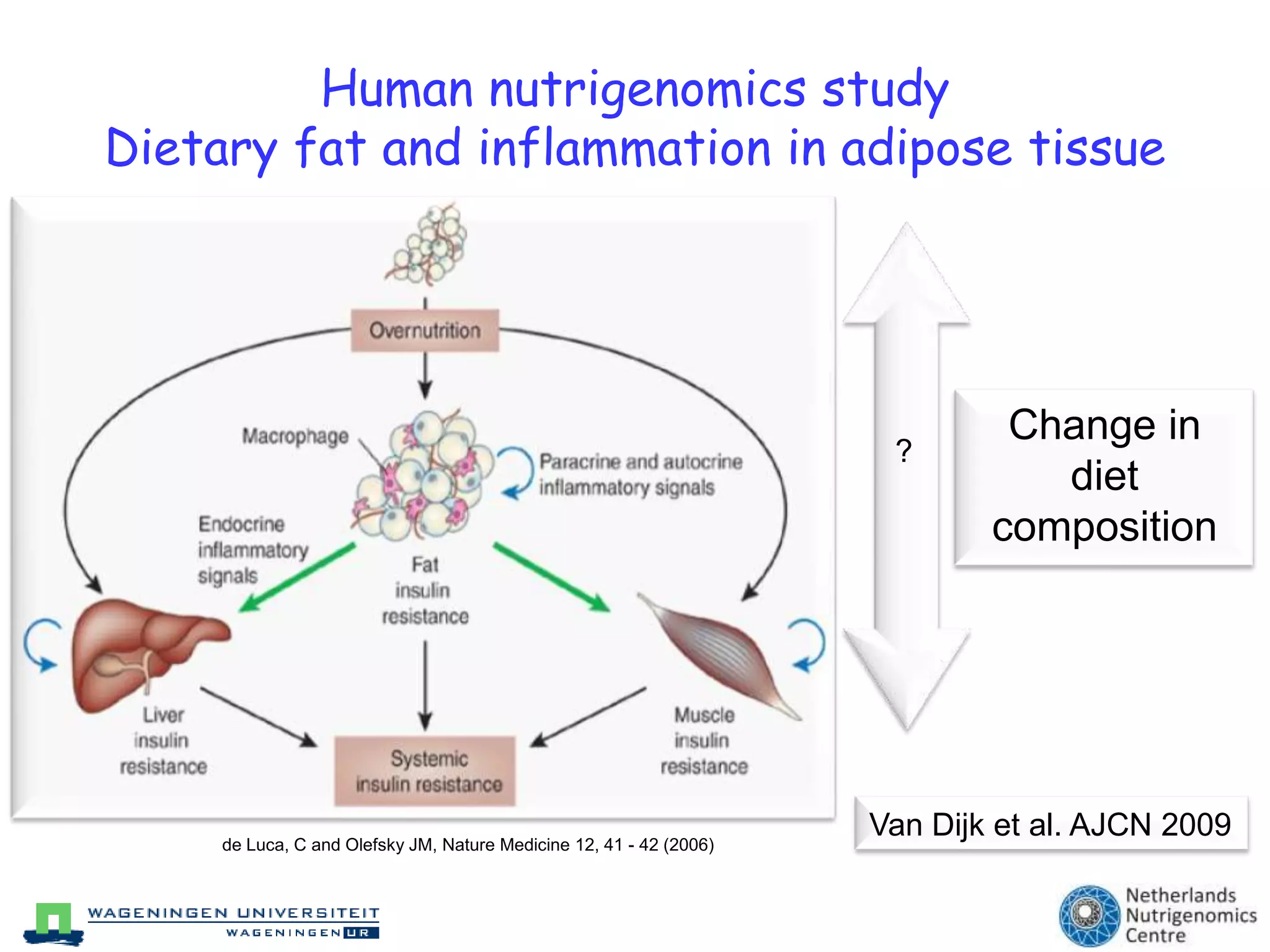
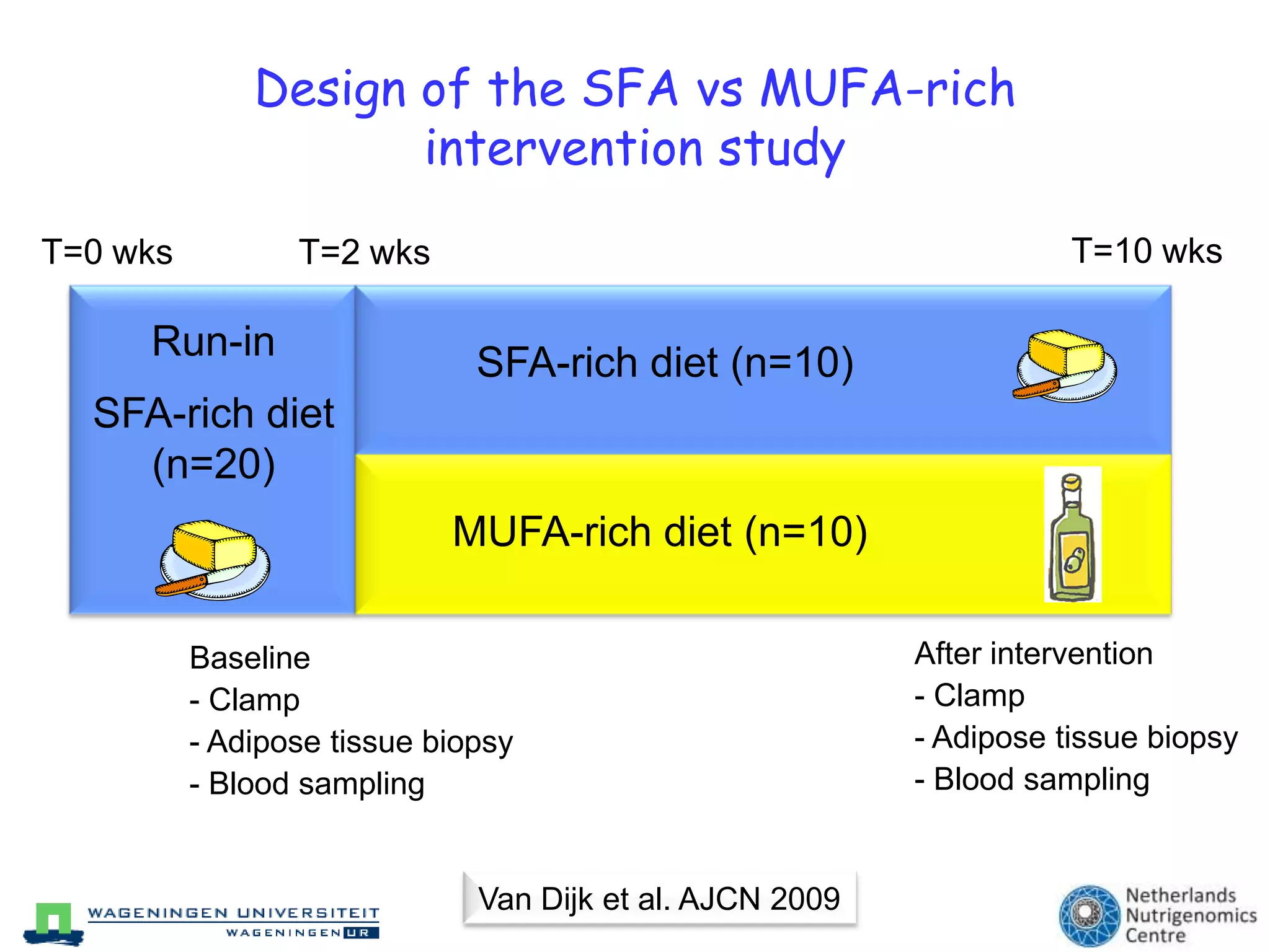
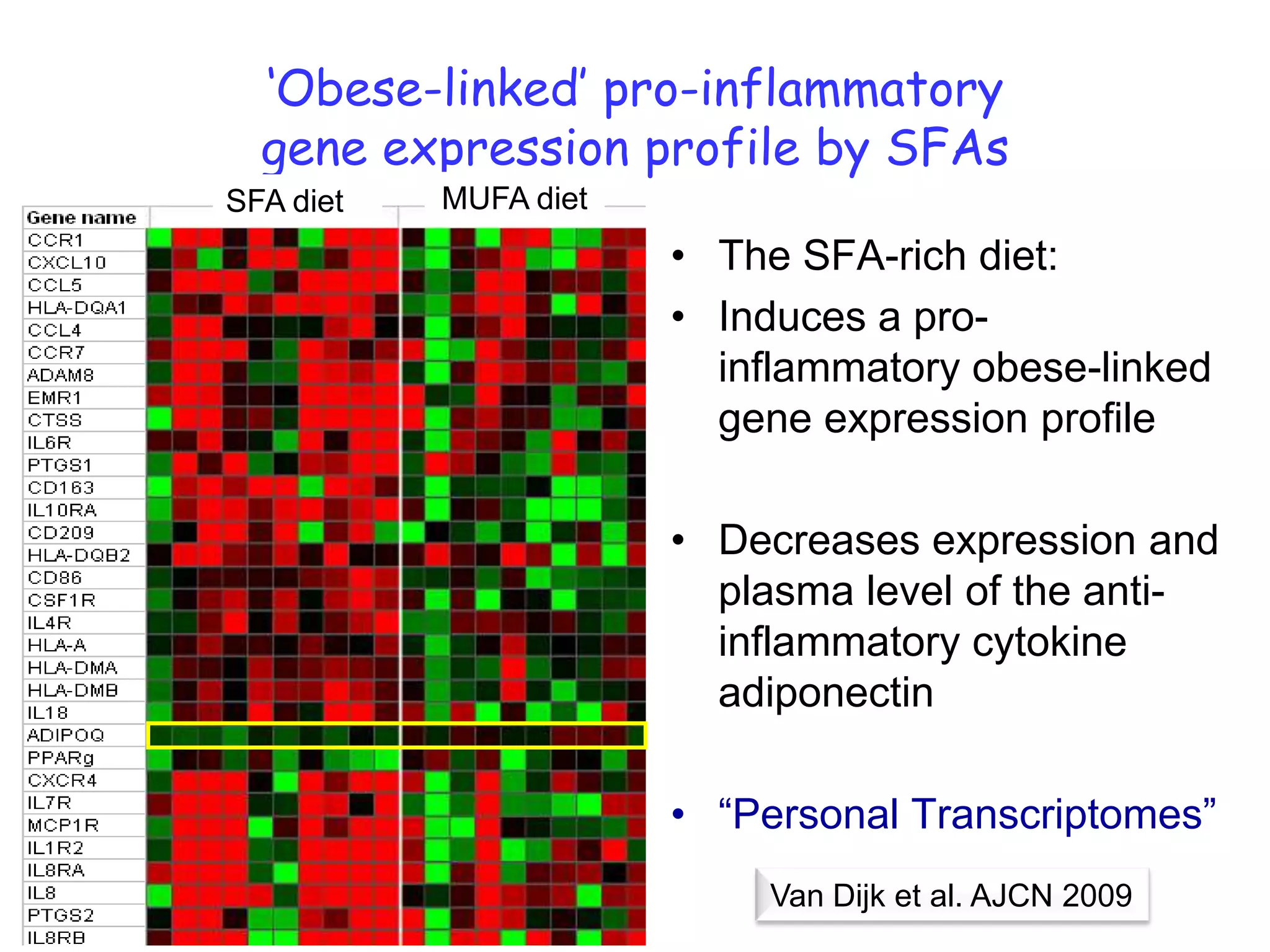
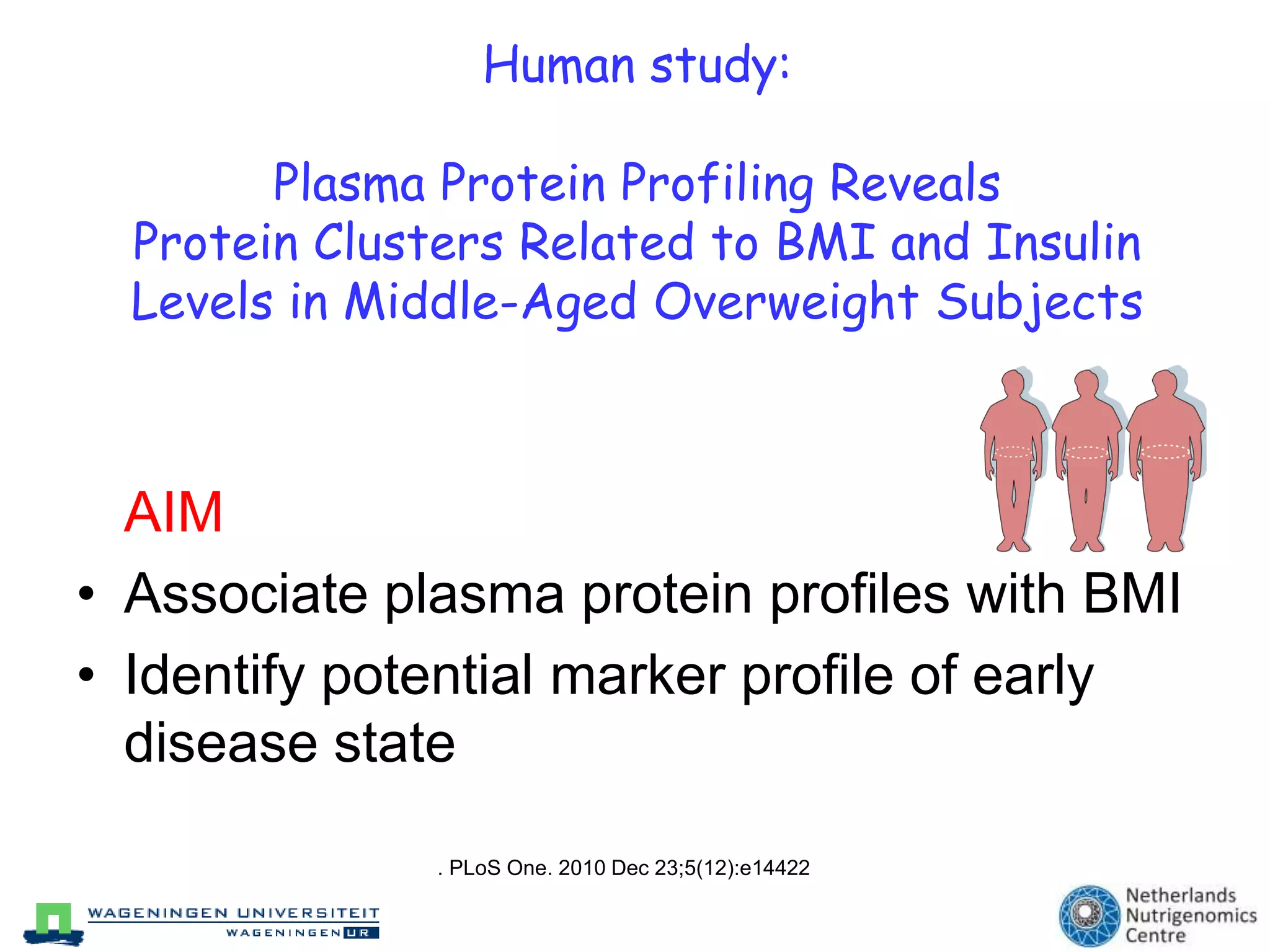
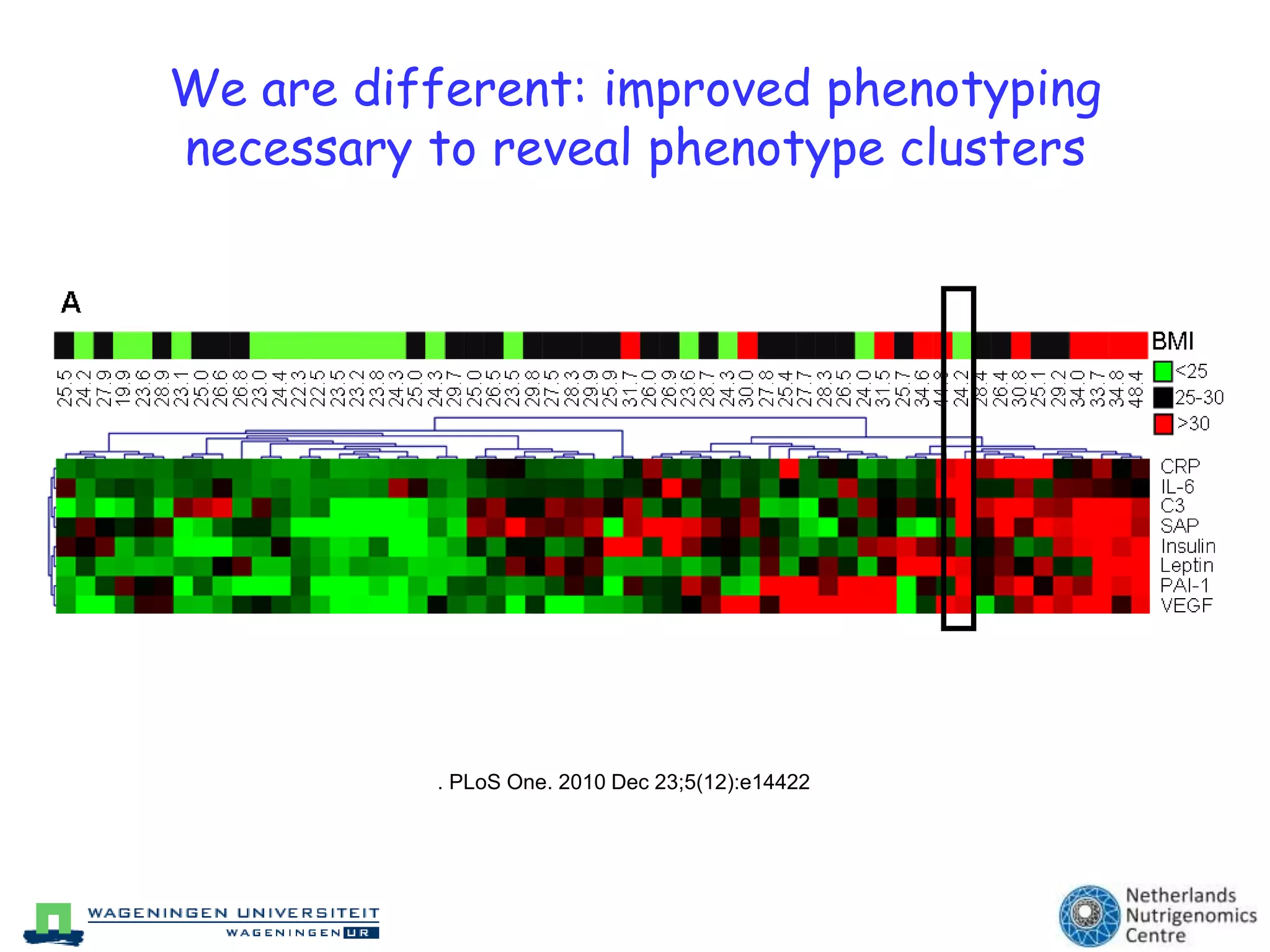
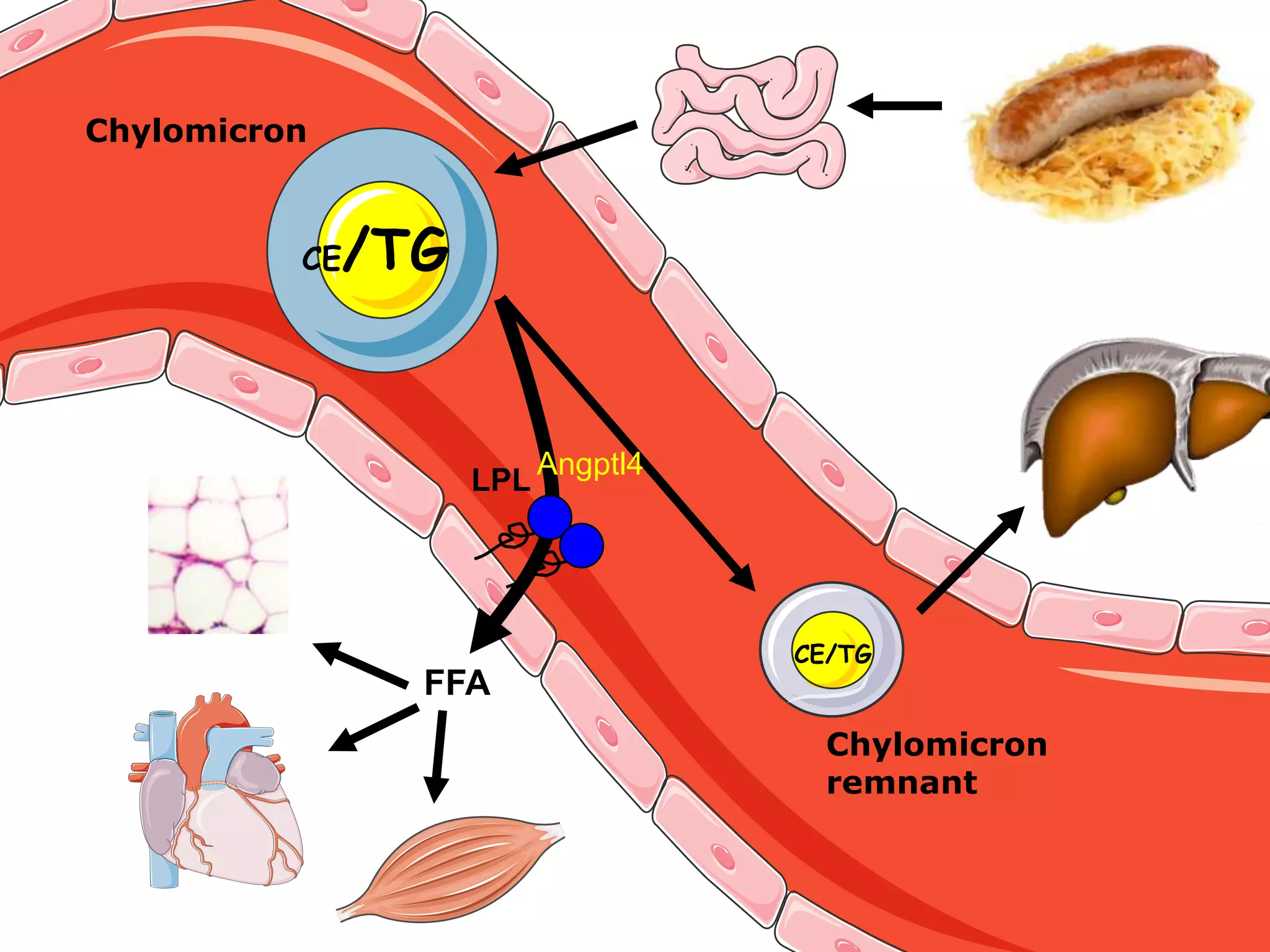
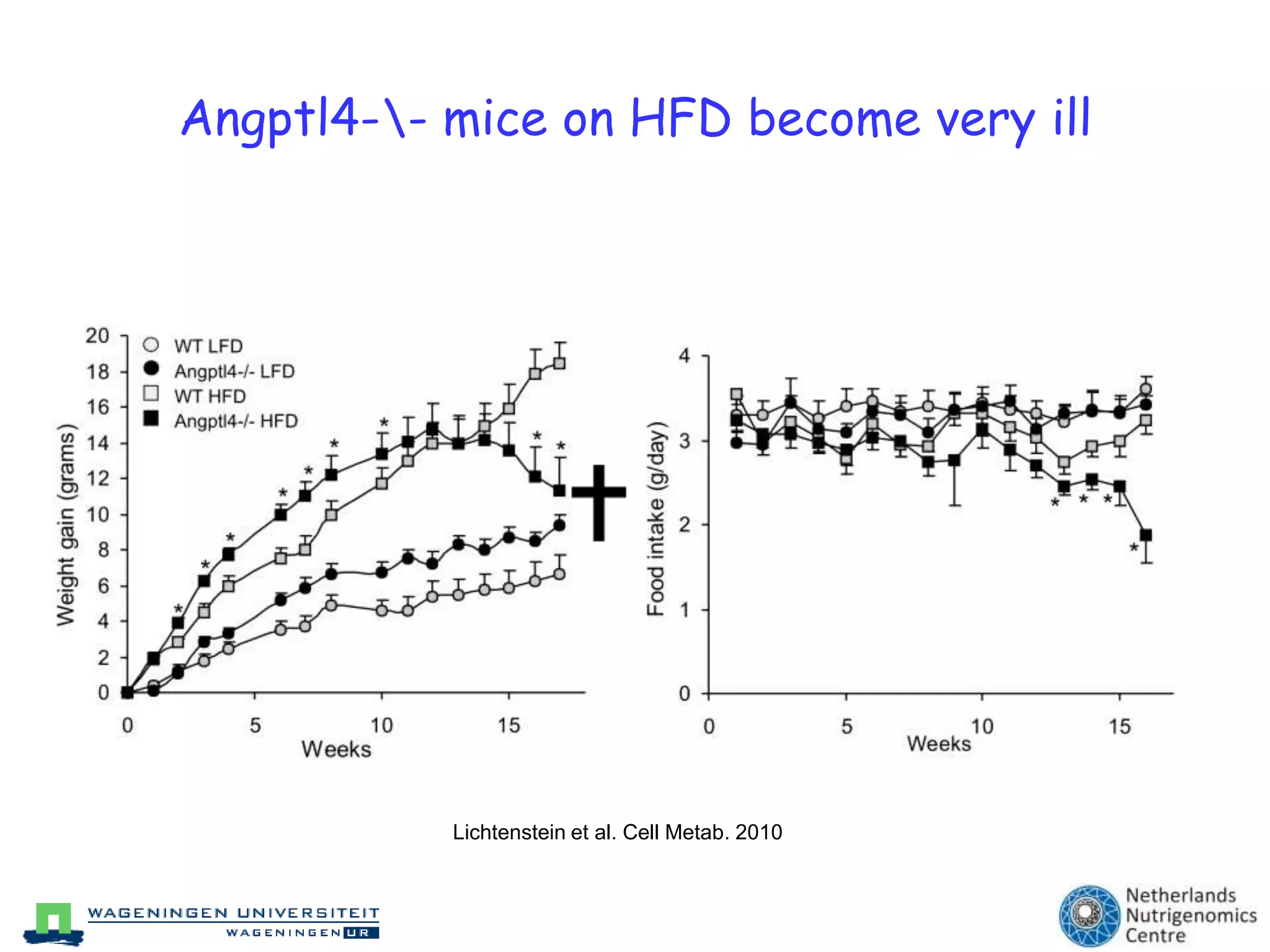
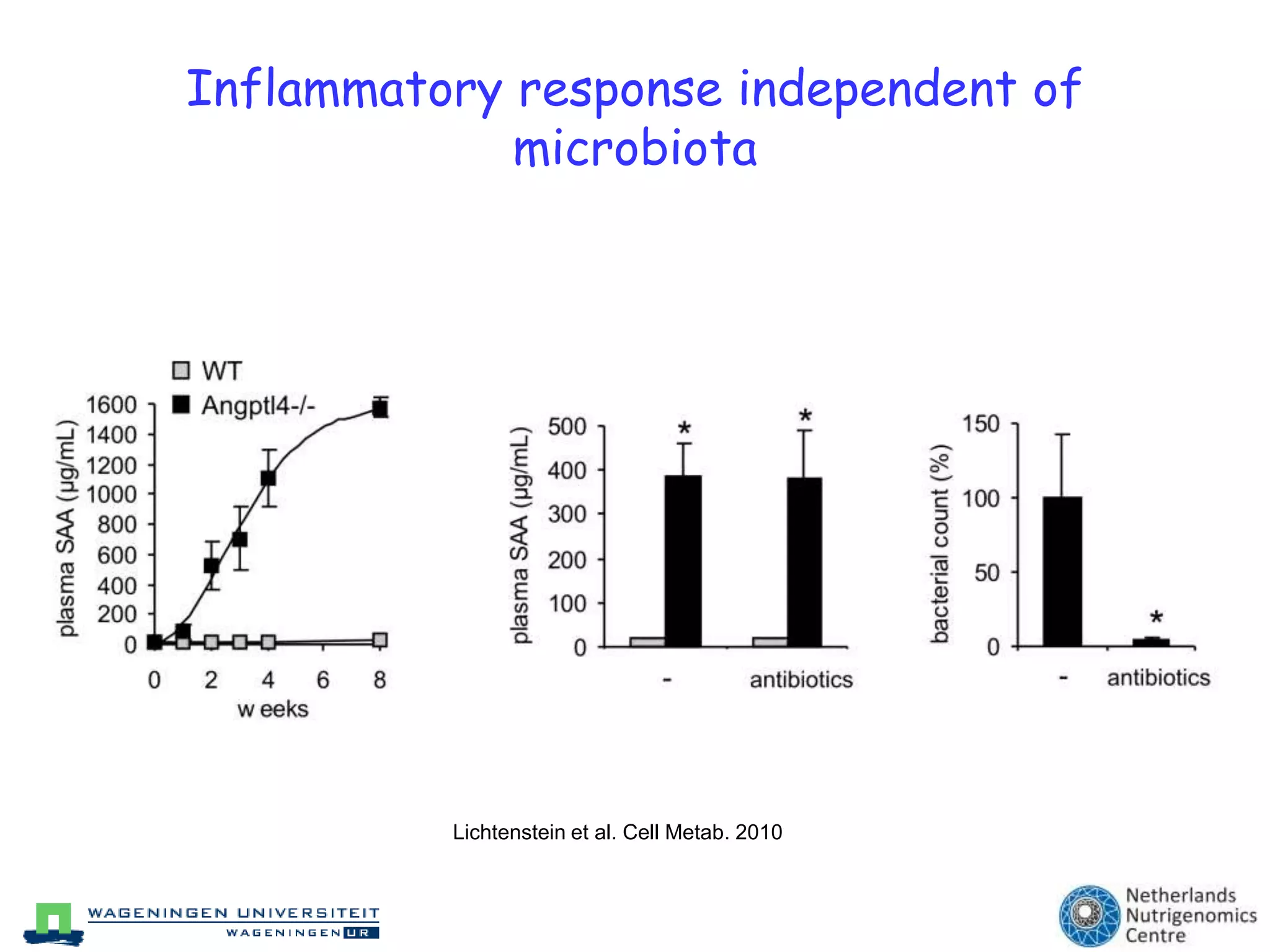
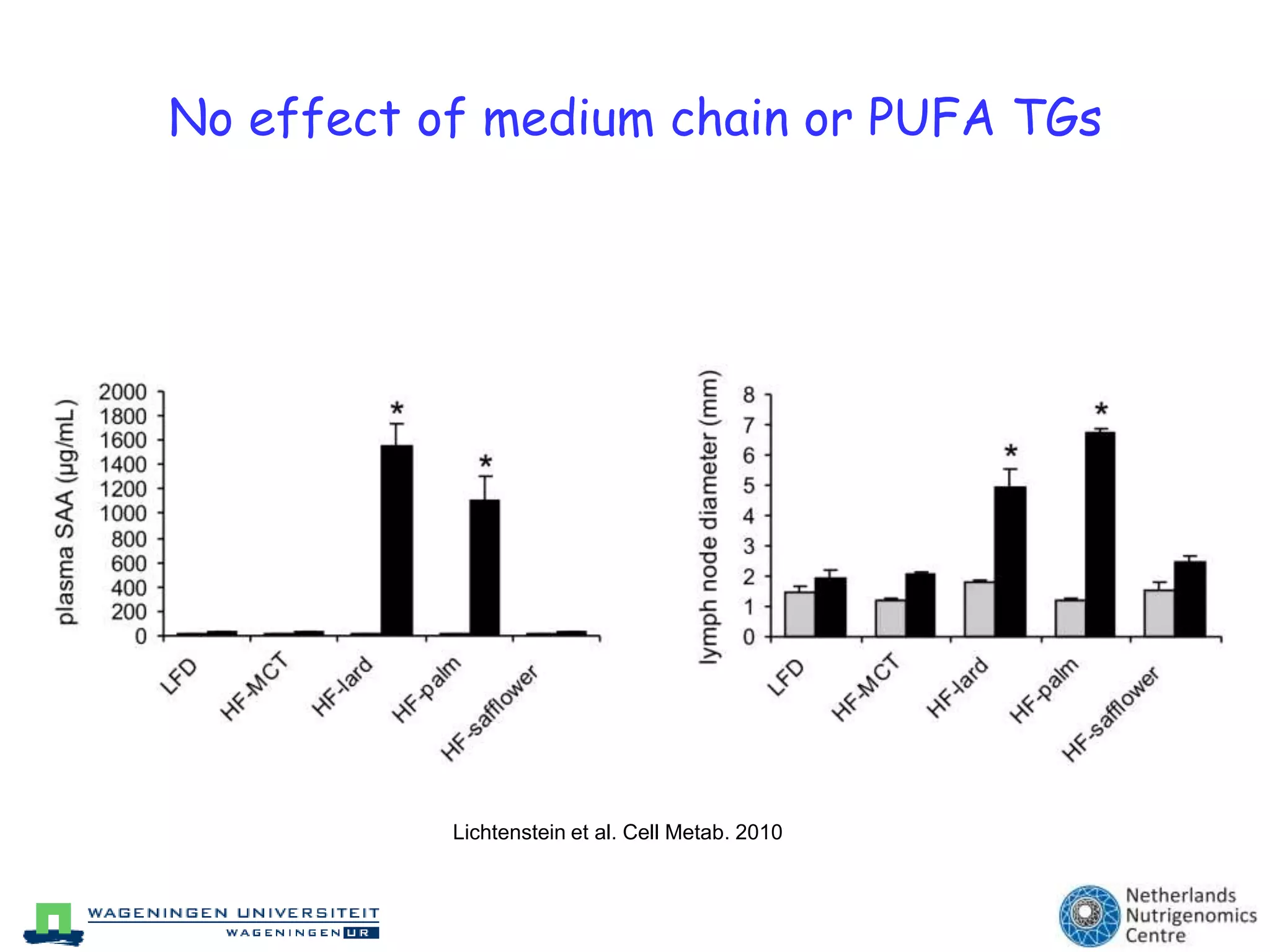
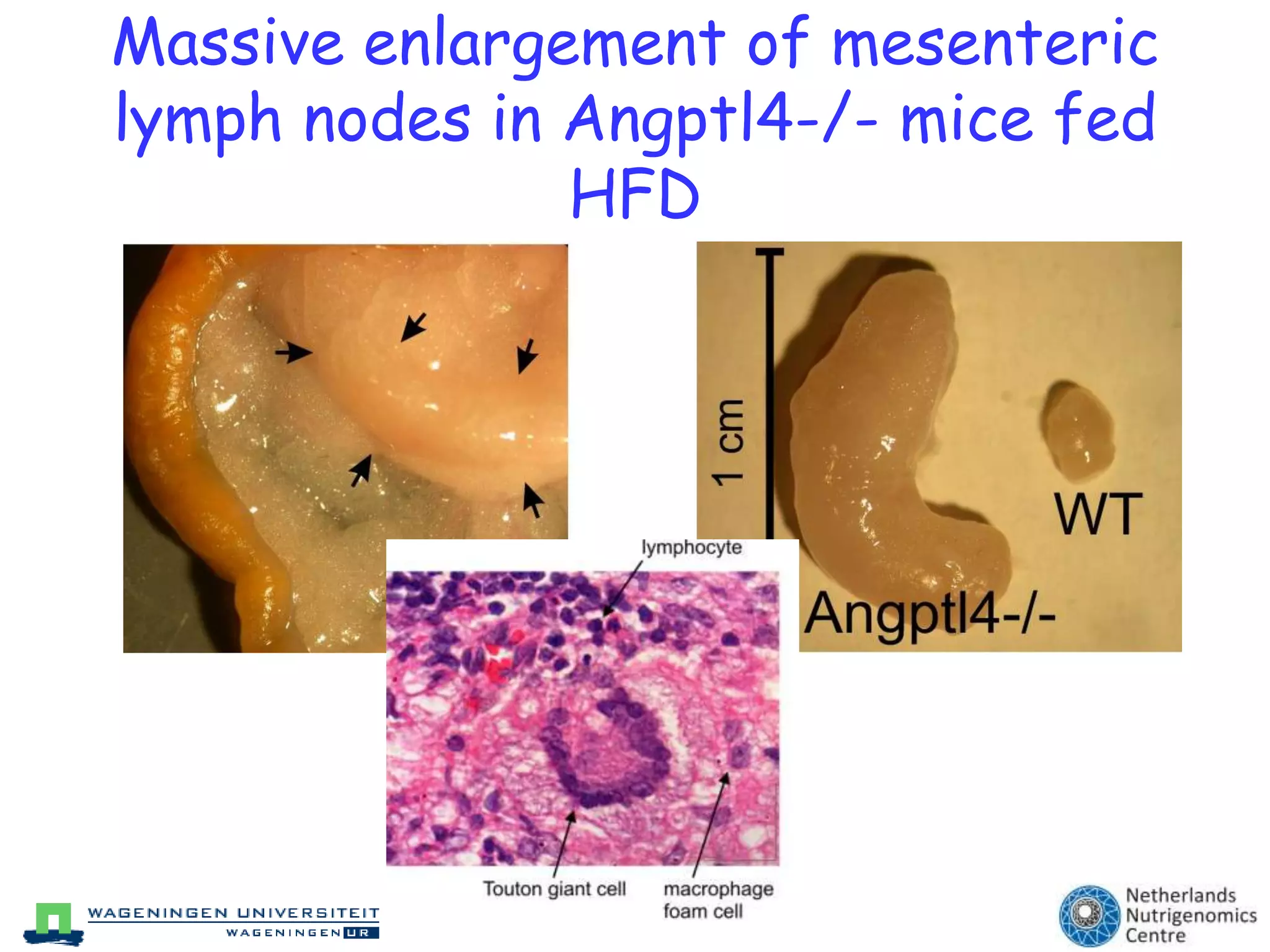
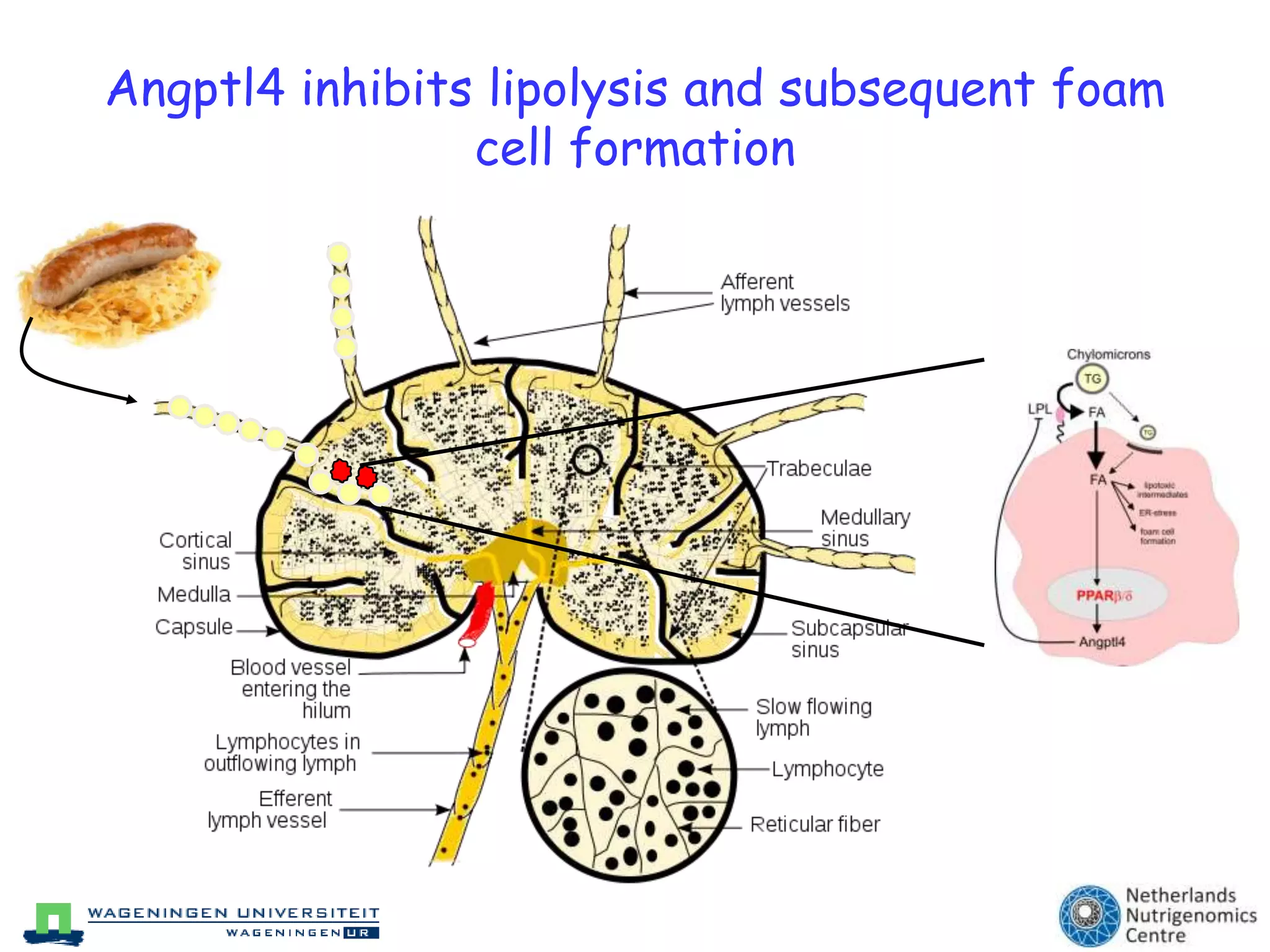
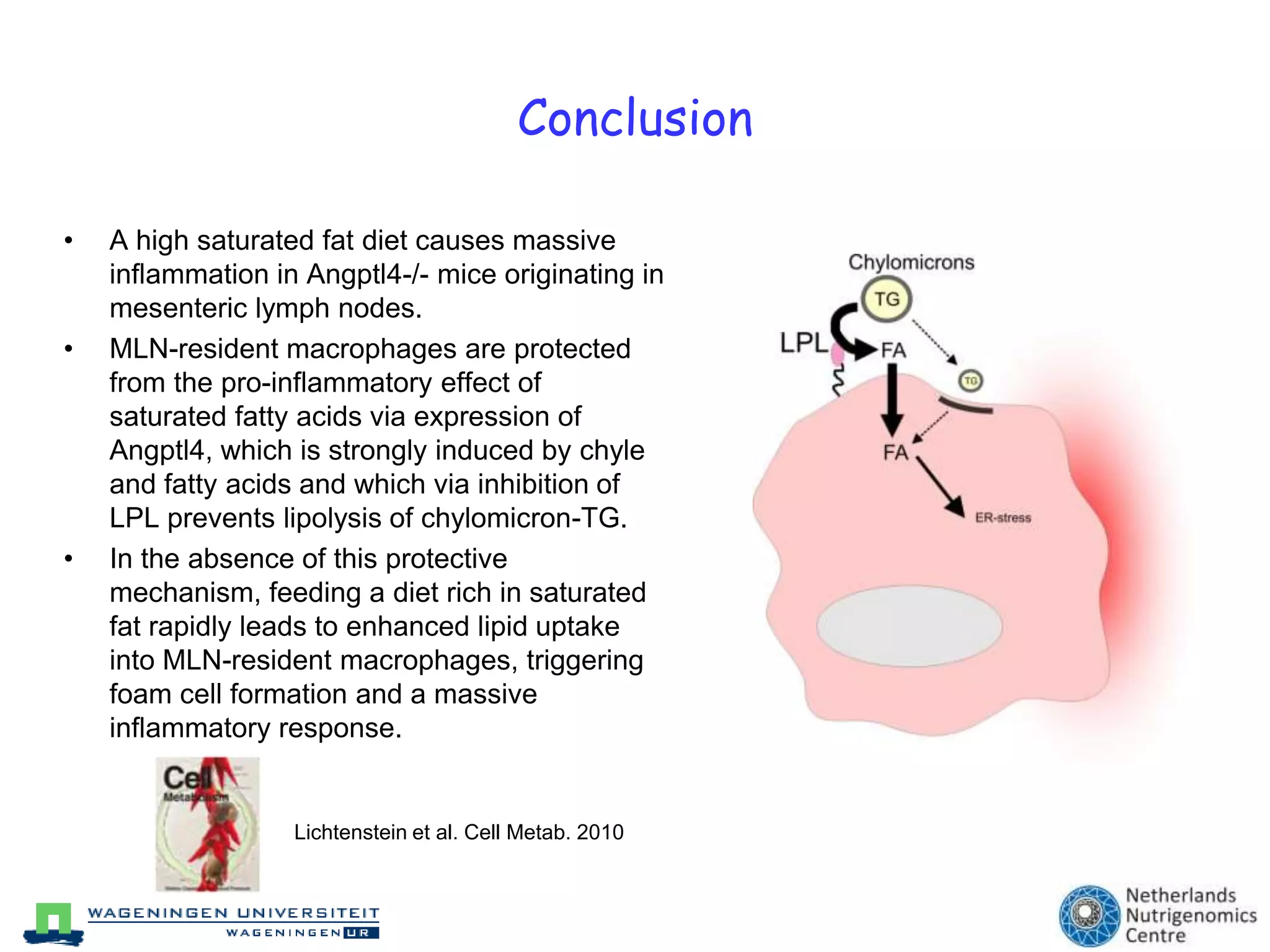
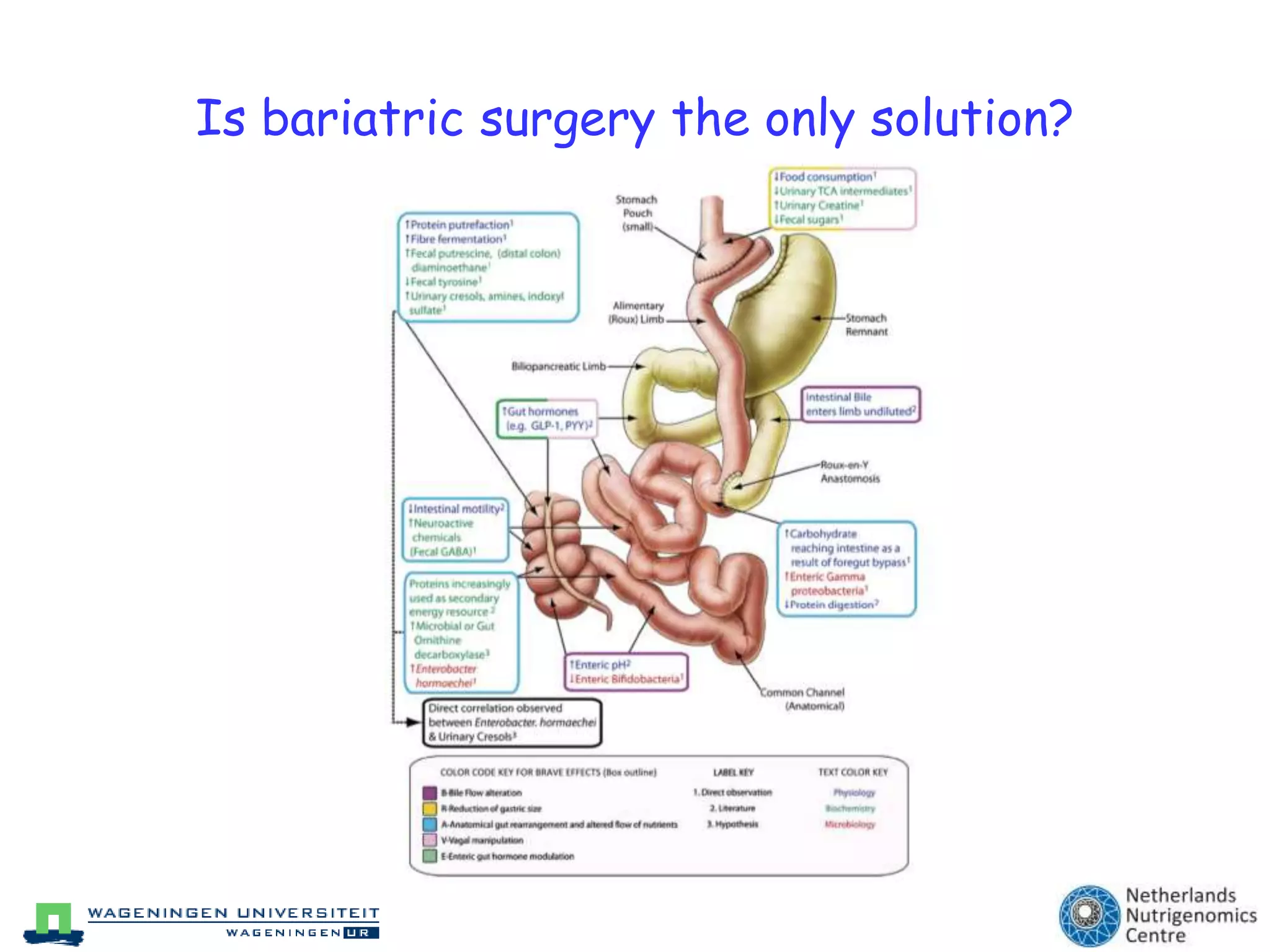
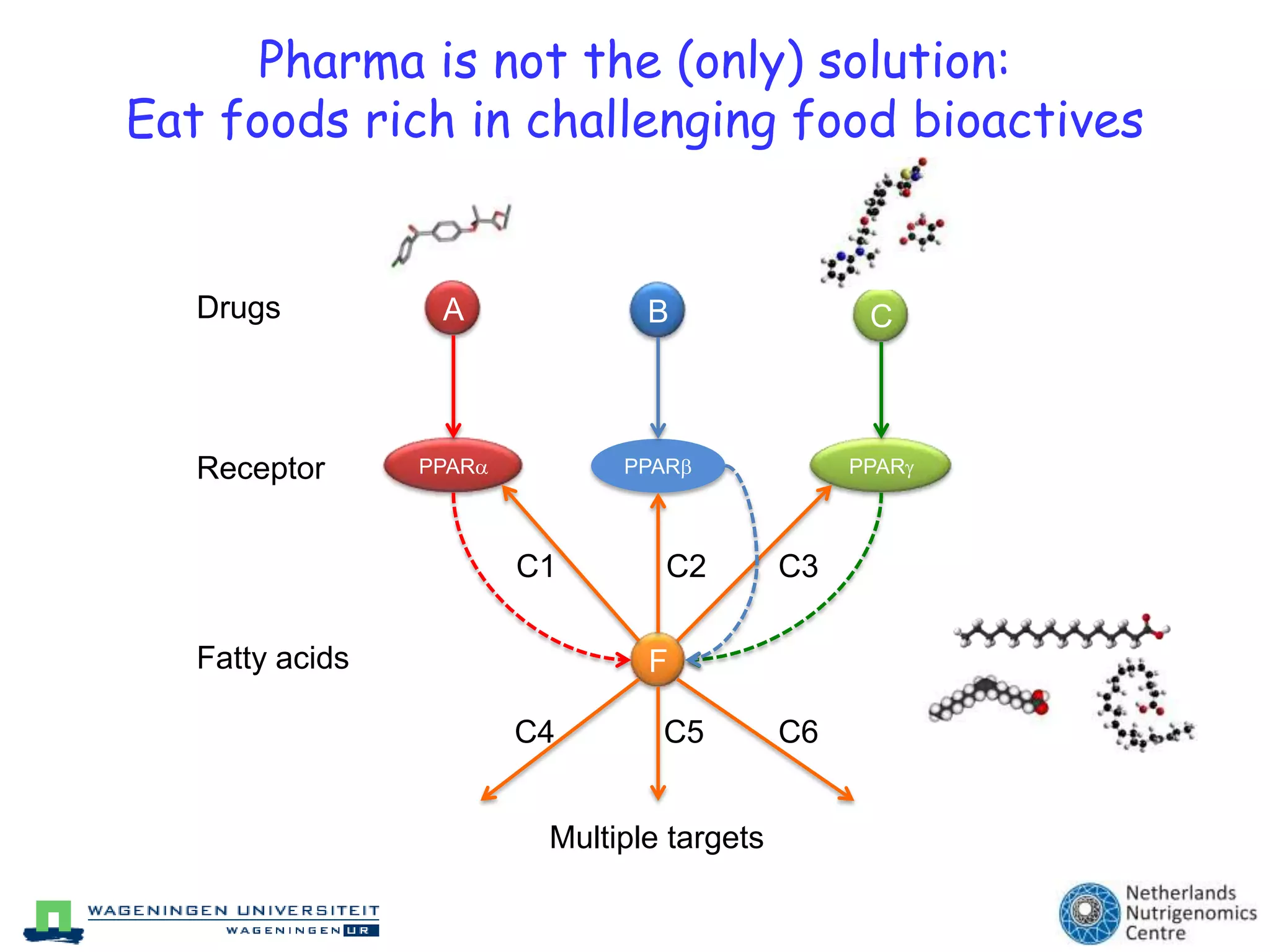
The document discusses the relationship between adipose tissue, innate immunity, inflammation, and the metabolic syndrome from a nutrigenomics perspective. It emphasizes the impact of dietary saturated fat on inflammation and health, outlining the characteristics of metabolic syndrome and proposing new nutritional science approaches. Additionally, the findings suggest a strong link between adipose tissue dysfunction and the progression of nonalcoholic fatty liver disease (NAFLD), highlighting potential early biomarkers for disease prediction.