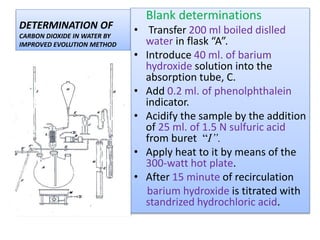
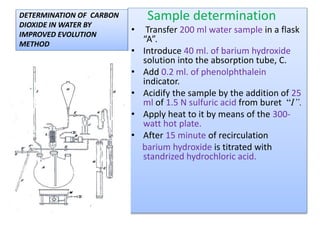
This document describes methods for determining the amount of carbon dioxide in water samples. It discusses how surface waters typically contain less than 10 ppm of free carbon dioxide while ground waters often exceed this level. The classical buret titration method and improved evolution method are described for determining carbon dioxide concentration. An instrumental coulometry method is also mentioned. The document provides details on reagents, procedures, and precautions for the buret titration method, which involves titrating a water sample with sodium hydroxide and measuring the amount of carbon dioxide absorbed.