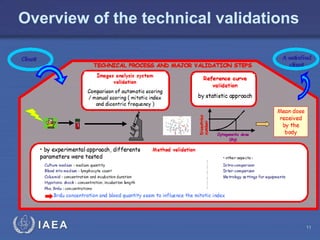
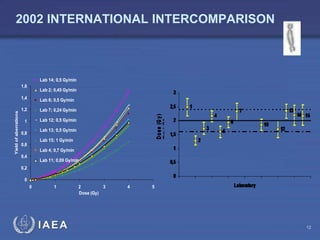
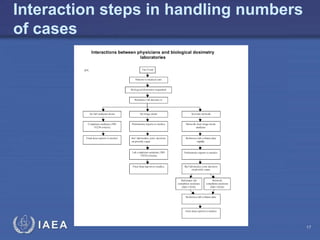
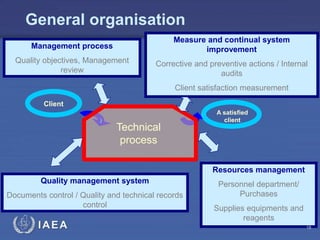
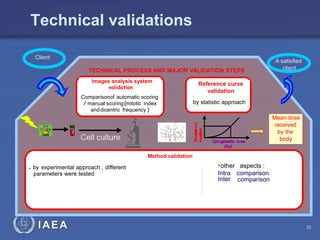
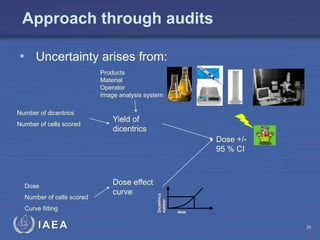
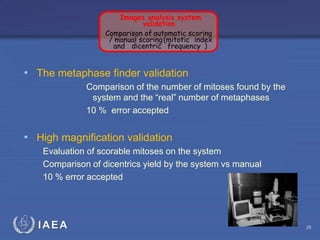
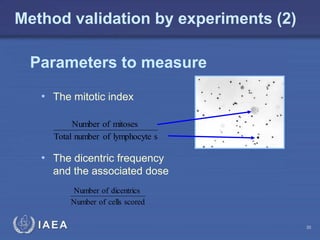
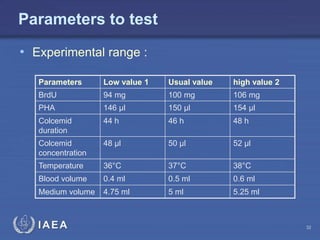
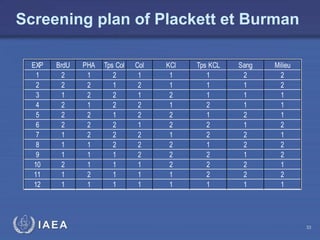
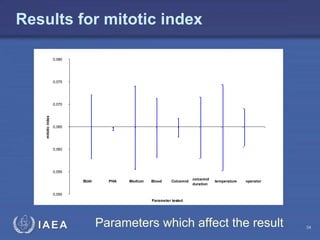
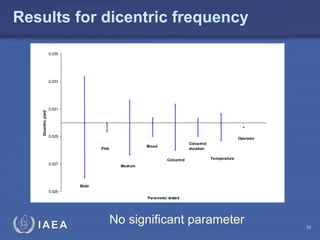
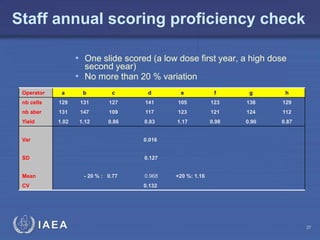
This document discusses quality assurance programs for cytogenetic dosimetry laboratories. It outlines the importance of ensuring staff safety, quality of results, and conforming to regulations. Specific risks from blood, UV light, and chemicals are highlighted. Infection control and proper use of equipment are emphasized. Standards like ISO are described which provide guidelines for documentation, validation, auditing, and continual improvement. Key aspects of the technical process like dose response curves, intercomparisons, and image analysis validation are summarized. Factors influencing dicentric yields are tested to validate the method and identify significant parameters. The importance of training and competency of operators is stressed.