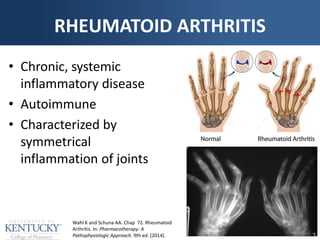
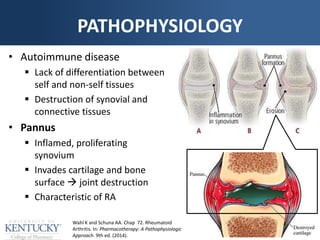
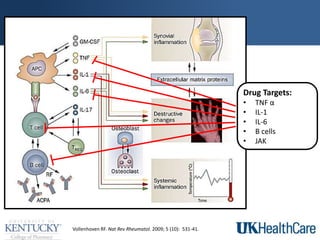
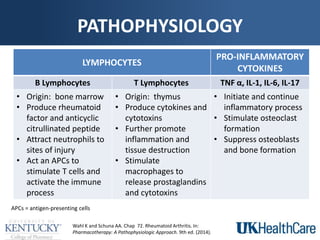
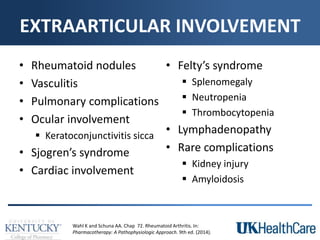
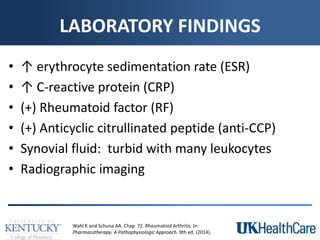
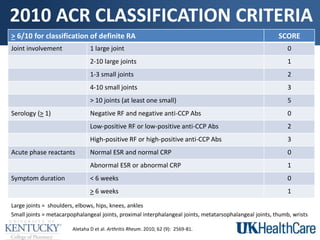
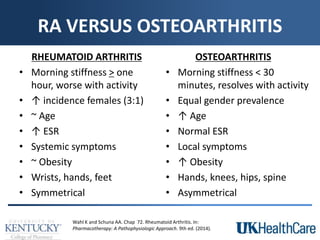
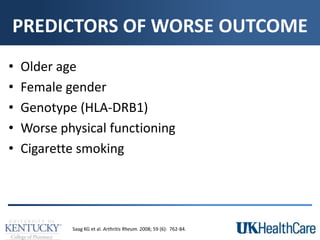
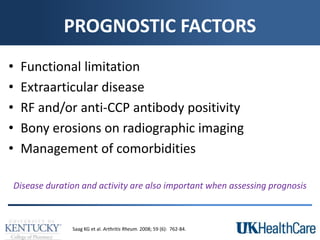
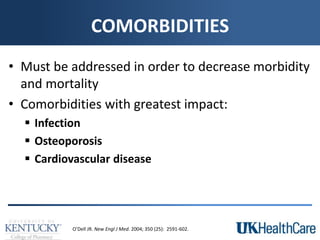
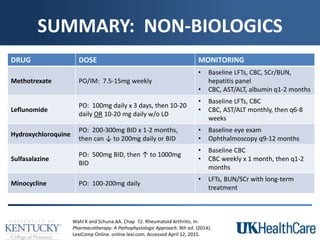
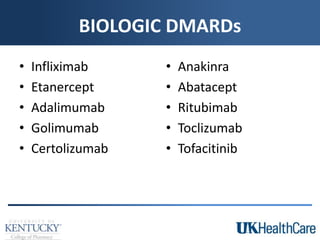
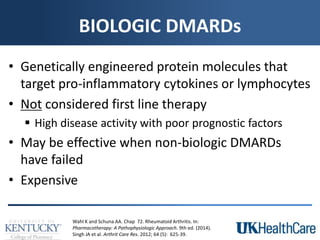
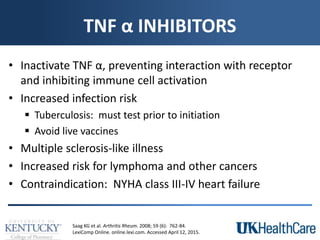
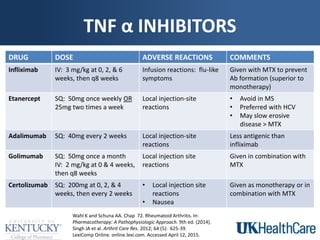
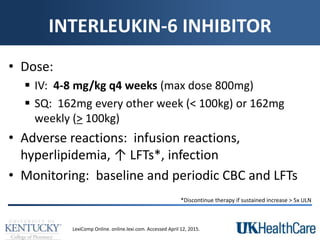
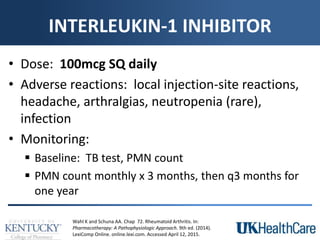
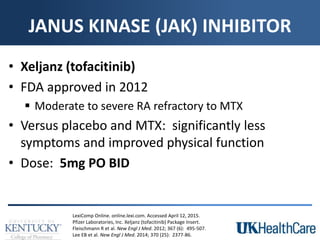
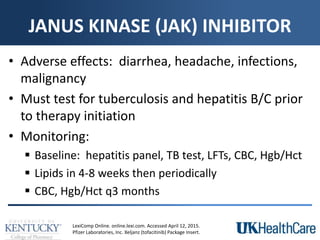
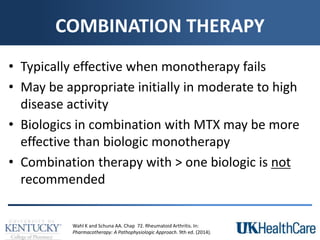
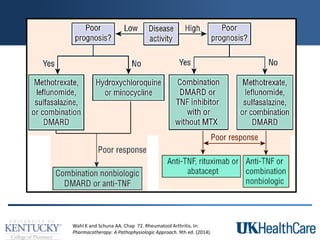
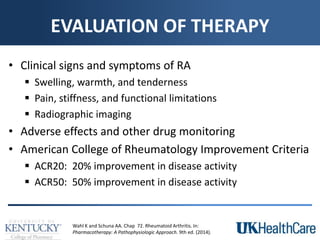
The document provides a comprehensive overview of rheumatoid arthritis (RA), detailing its characteristics, epidemiology, pathophysiology, clinical presentation, diagnosis, and treatment options. It emphasizes the importance of individualized therapeutic regimens that may include non-pharmacologic and pharmacologic therapies, specifically disease-modifying antirheumatic drugs (DMARDs). The summary also highlights the need for monitoring and management of comorbidities to improve patient outcomes.