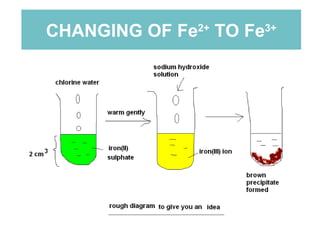
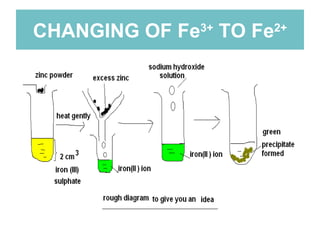
The document summarizes several chemistry experiments involving redox reactions:
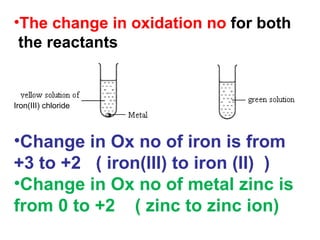
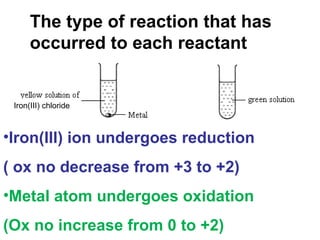
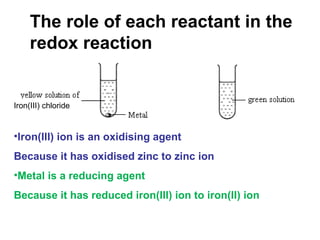
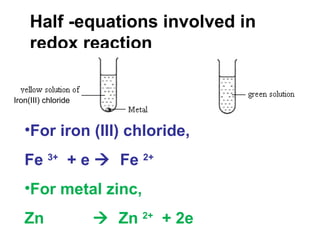
1) An experiment where iron(III) chloride reacts with zinc, producing a green solution containing iron(II) ions. Zinc undergoes oxidation and iron(III) undergoes reduction in this redox reaction.
2) An experiment verifying that iodide ions act as a reducing agent by reducing acidified potassium manganate(VII) in a galvanistic cell containing a U-tube filled with sulfuric acid and electrodes.
3) Suggestions that sodium, potassium or lithium could be the metal M that reacts with oxygen to form an alkaline solution, along with the half reactions for this oxidation and

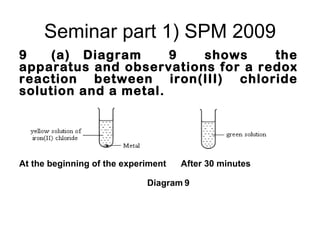

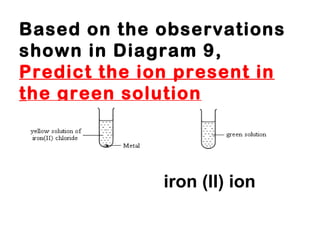






![Part 1)SPM 2008 9 (a) A metal M reacts with oxygen to form an oxide. The oxide is very soluble in water to produce an alkaline solution. Suggest the identity of metal M and describe an observation when the metal you have named reacts with oxygen, write the half-equations for oxidation and reduction for the reaction. [4 marks]](https://image.slidesharecdn.com/campsciencejuly2011part1-110722065425-phpapp01/85/Revision-on-redox-july-2011-part-1-15-320.jpg)

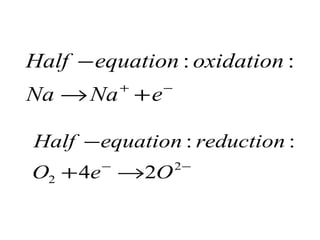
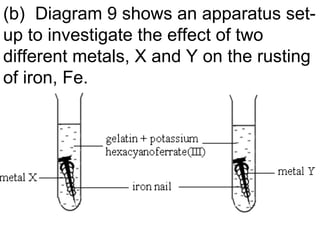

![Based on Table 9, suggest the identity of metals, X and Y. Give two reasons for each of your choices [6 marks] Dark blue colour shows presence of iron(II) ions. Means iron nail has rusted Metal X is copper/ any metals below iron in ECS Because copper is less electropositive than iron So, copper will encourage iron to rust](https://image.slidesharecdn.com/campsciencejuly2011part1-110722065425-phpapp01/85/Revision-on-redox-july-2011-part-1-20-320.jpg)
![Based on Table 9, suggest the identity of metals, X and Y. Give two reasons for each of your choices [6 marks] No change means iron nail has not rusted Metal Y is zinc/Magnesium Because zinc/magnesium is more electropositive than iron So, zinc/magnesium will protect iron from rusting](https://image.slidesharecdn.com/campsciencejuly2011part1-110722065425-phpapp01/85/Revision-on-redox-july-2011-part-1-21-320.jpg)

![Seminar Part 1) SPM 2008 9(C) Iron(II) ions can be converted to iron(III) ions and iron(III) ions can be converted back to iron(II) ions. By using a named metal as a reducing agent and a named halogen as an oxidising agent, describe briefly how you would carry out these two conversions. Describe a test to show that each conversion has taken place. [10 marks]](https://image.slidesharecdn.com/campsciencejuly2011part1-110722065425-phpapp01/85/Revision-on-redox-july-2011-part-1-23-320.jpg)