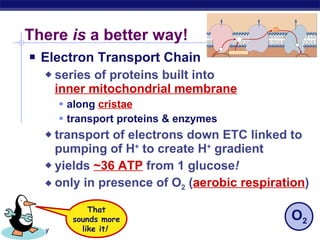
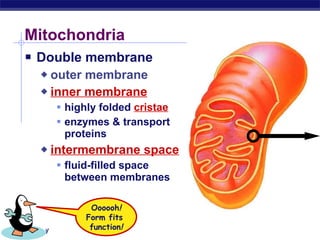
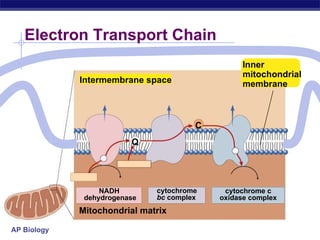
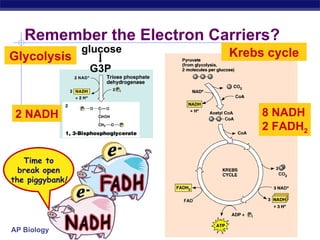
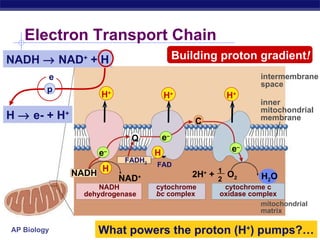
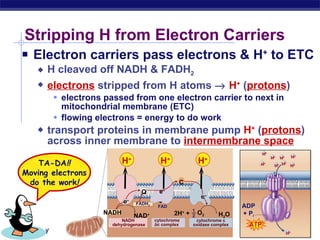
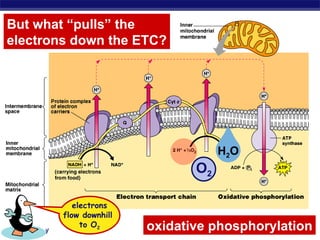
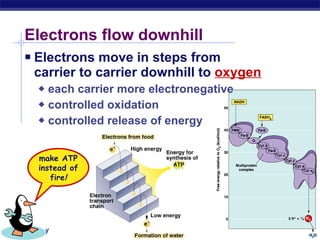
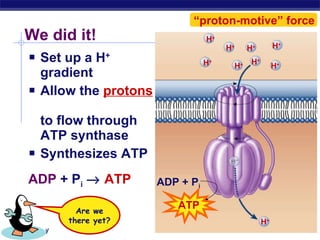
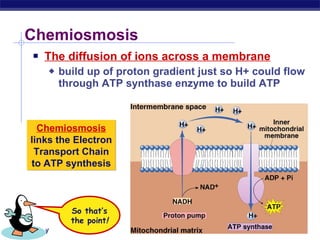
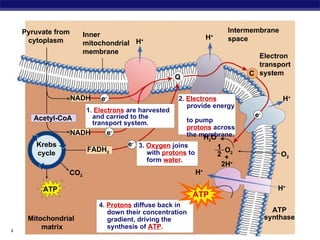
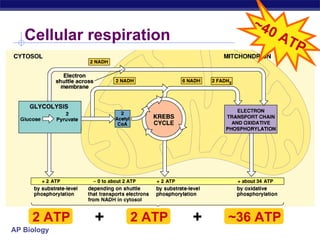
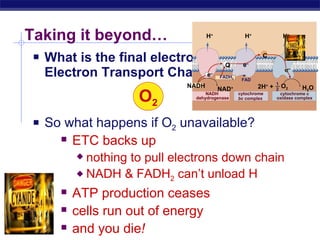
The document summarizes cellular respiration stage 4, the electron transport chain. The electron transport chain uses a series of protein transporters in the mitochondrial inner membrane to transport electrons and pump hydrogen ions out of the matrix. This creates a proton gradient that drives ATP synthase to generate approximately 36 ATP molecules from each glucose molecule. Oxygen is the final electron acceptor in the chain and its presence is required for aerobic respiration to occur.