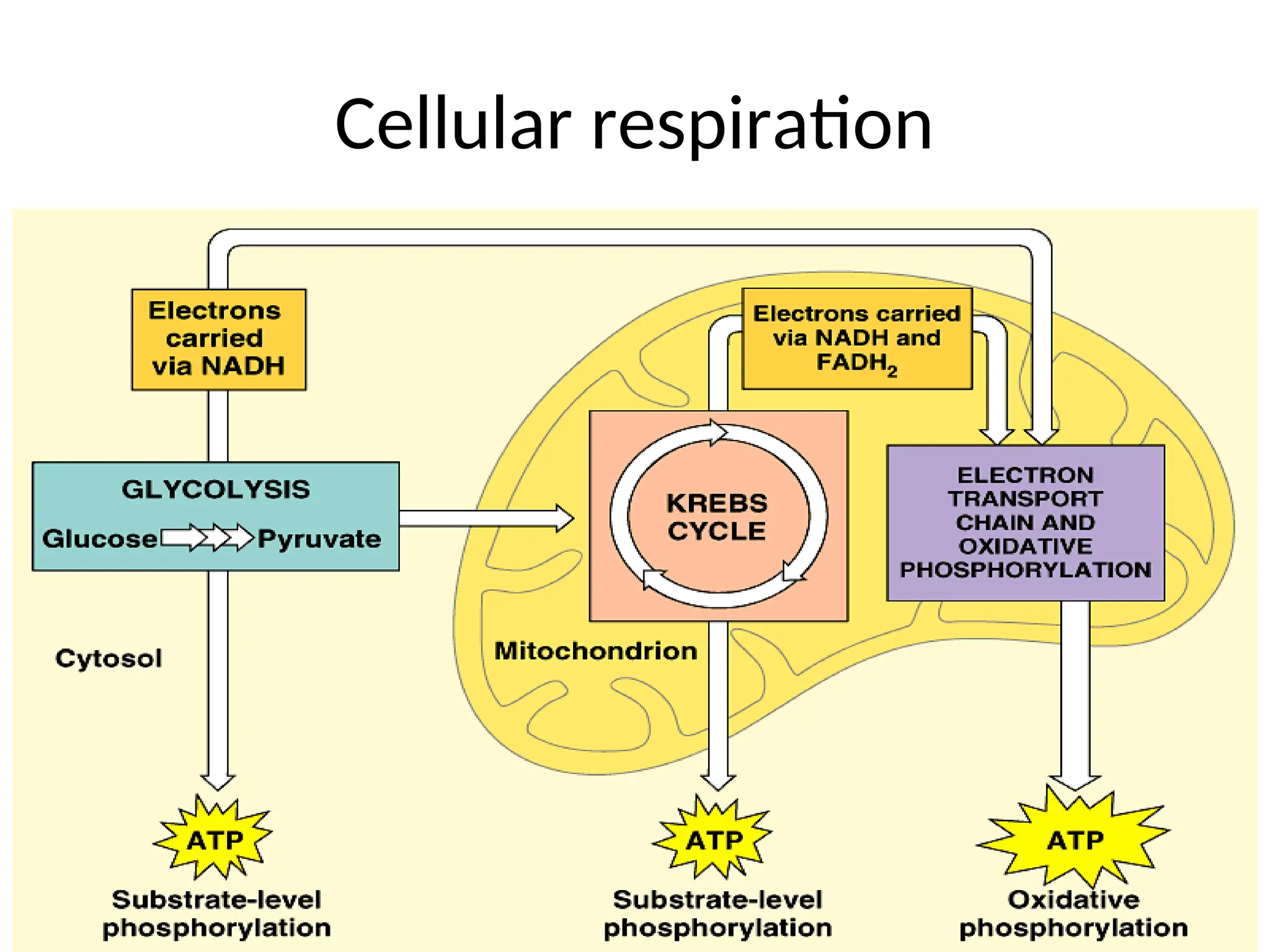
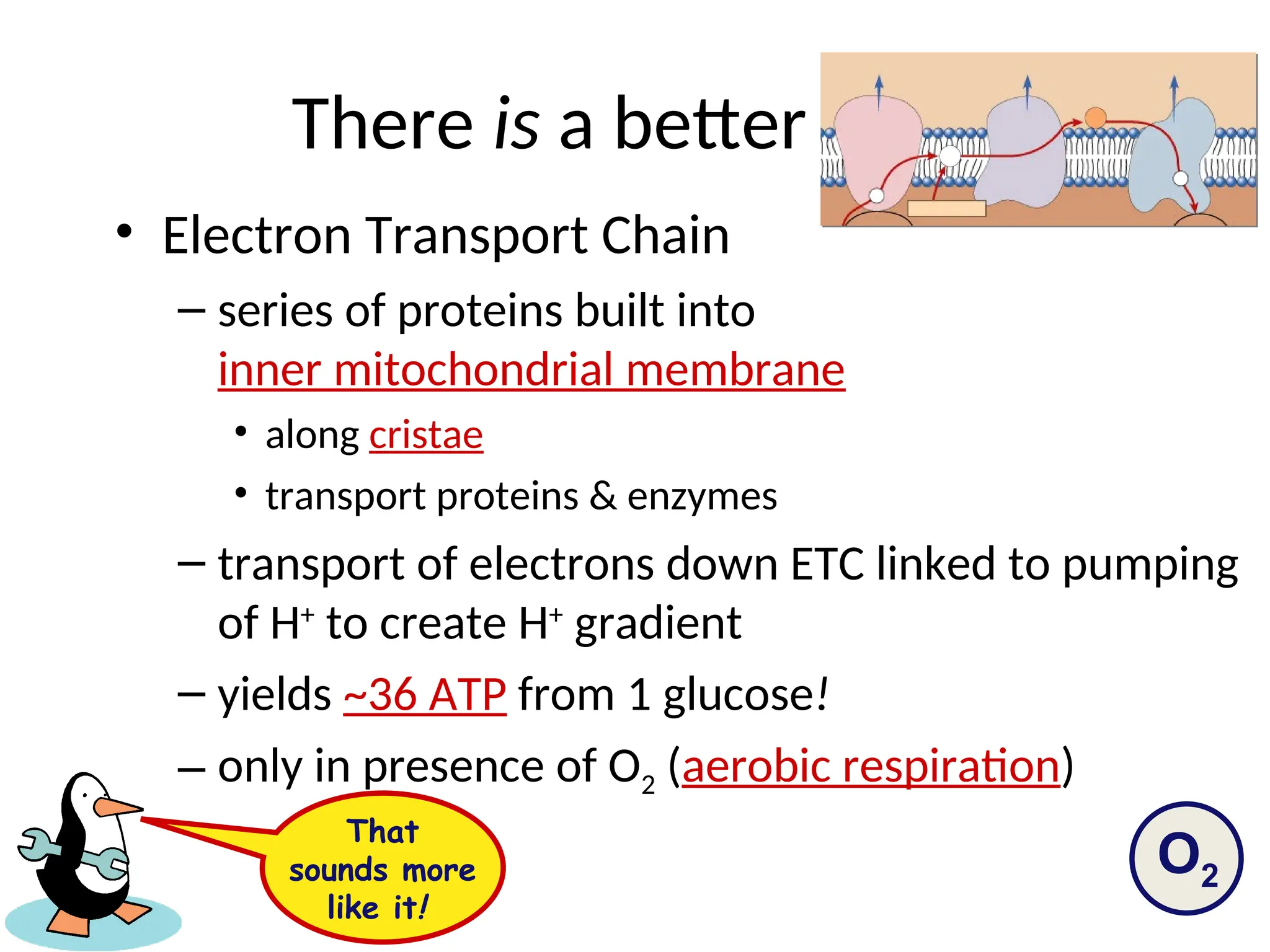
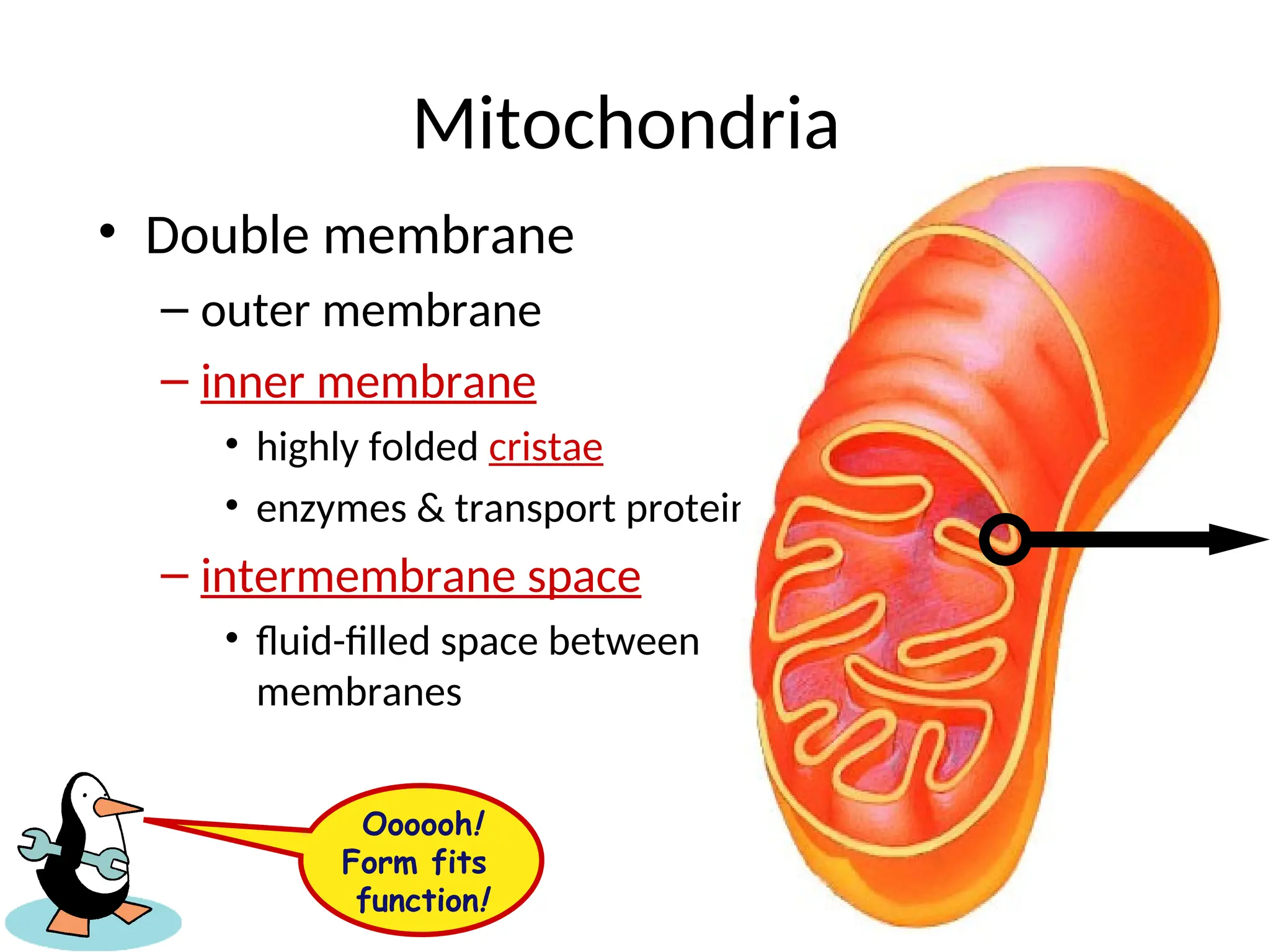
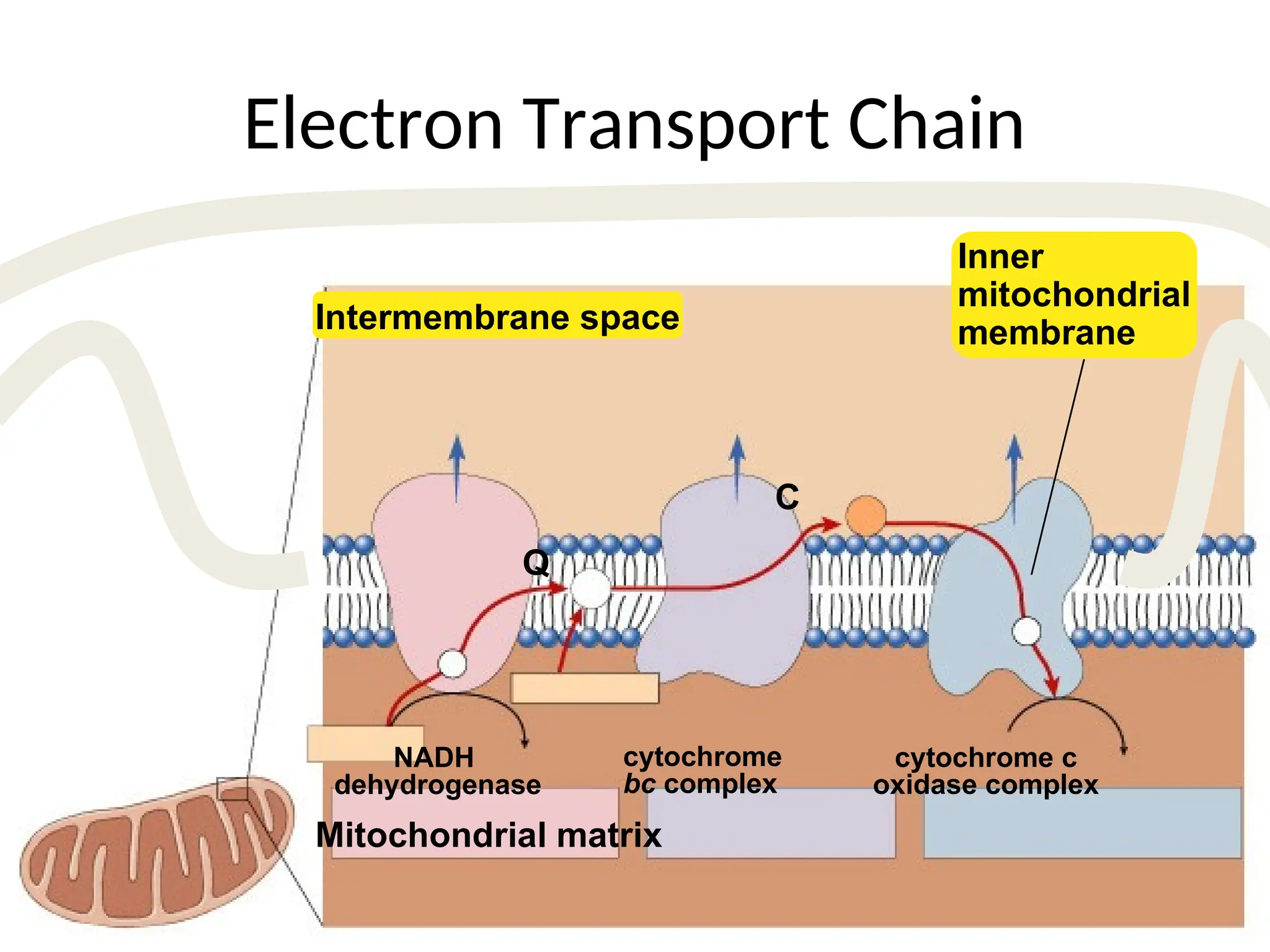
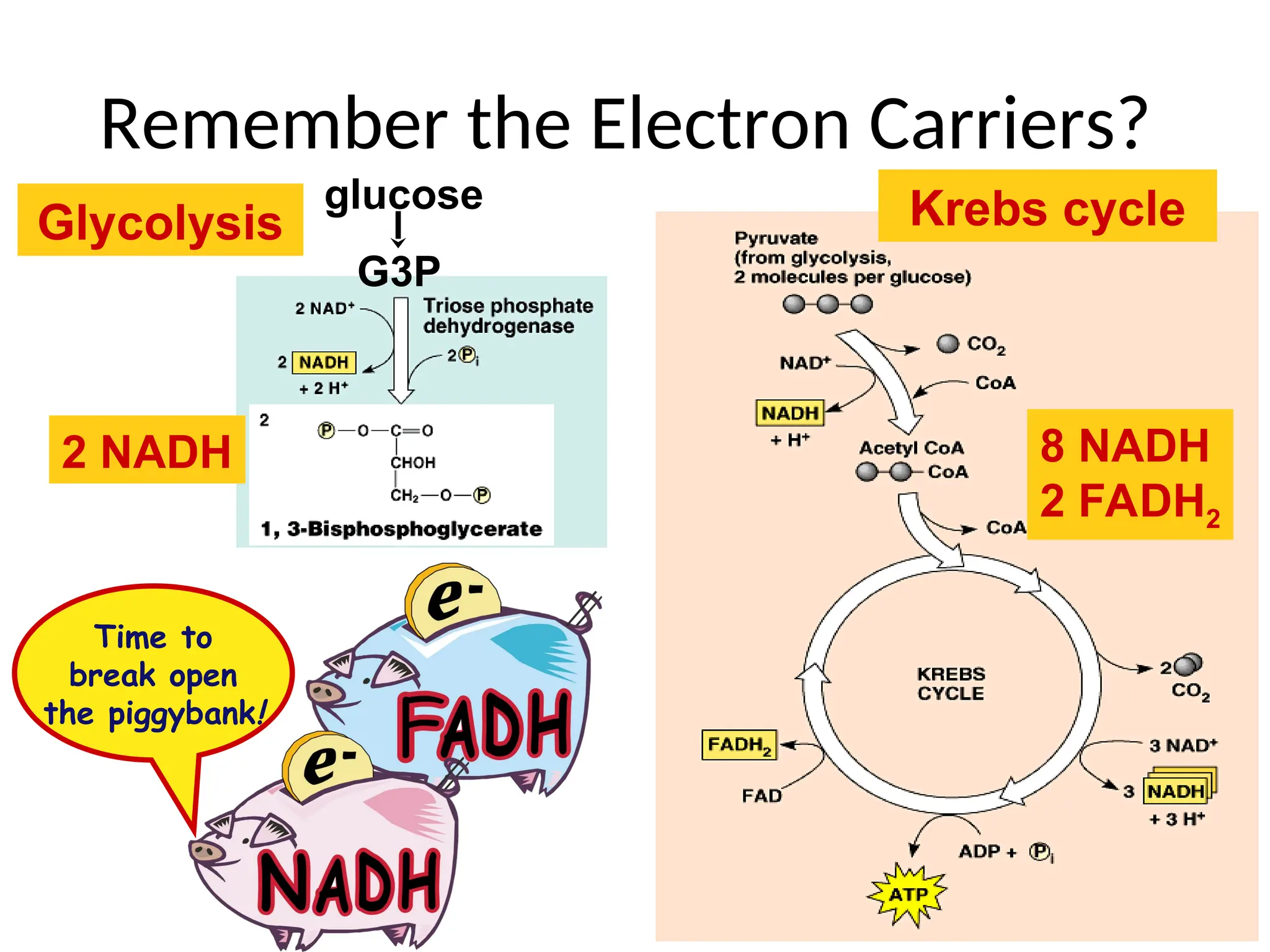
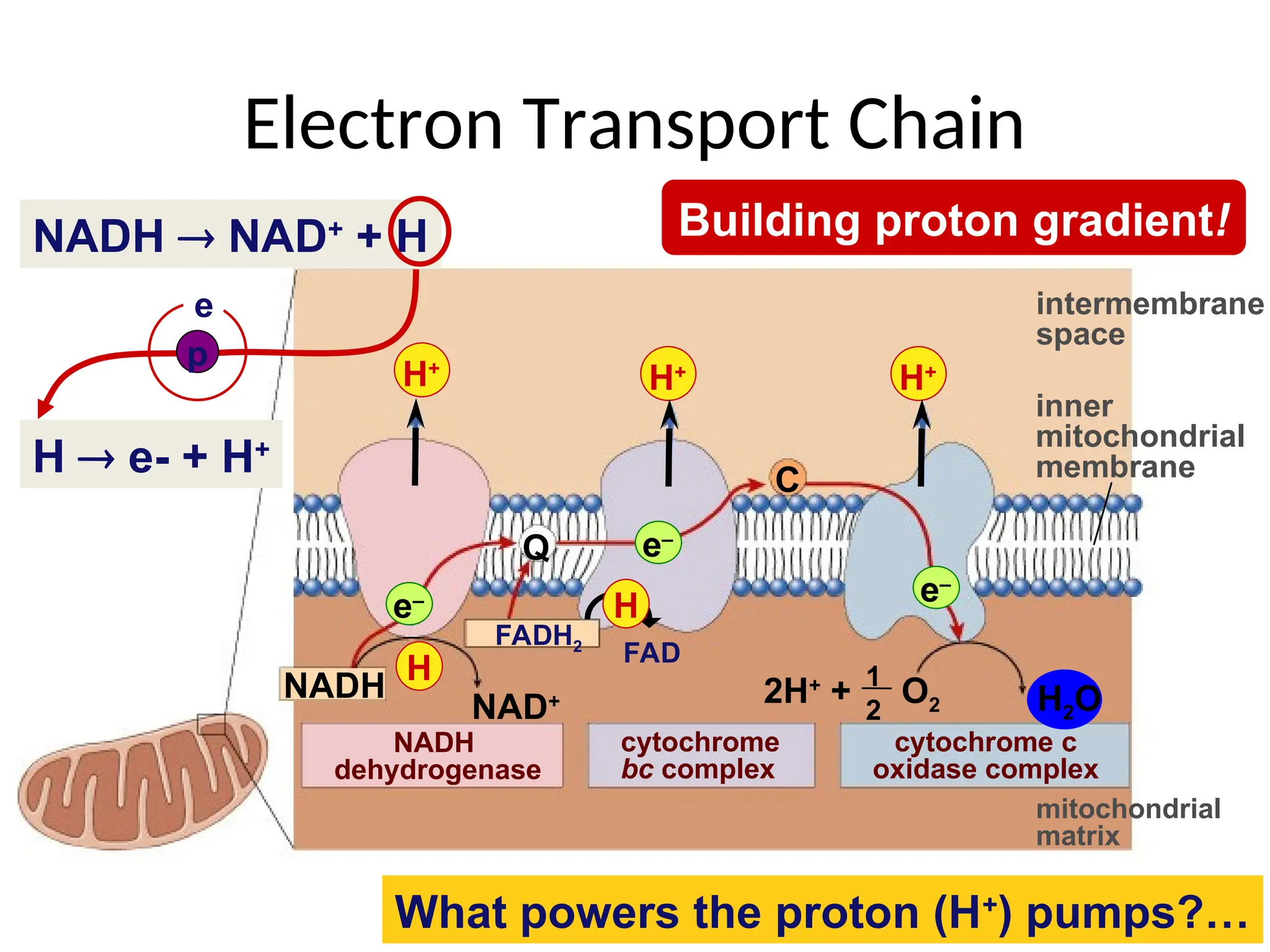
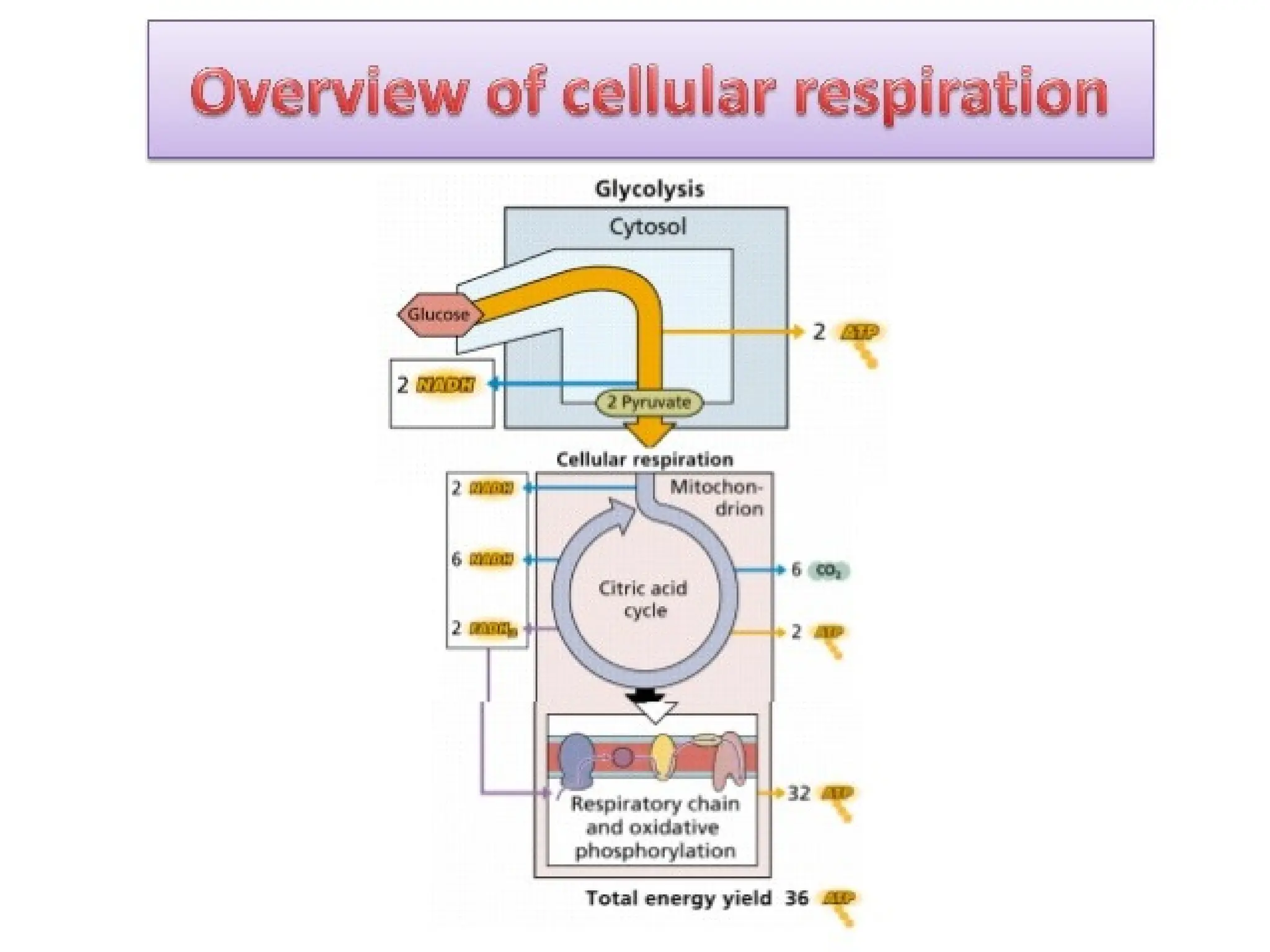
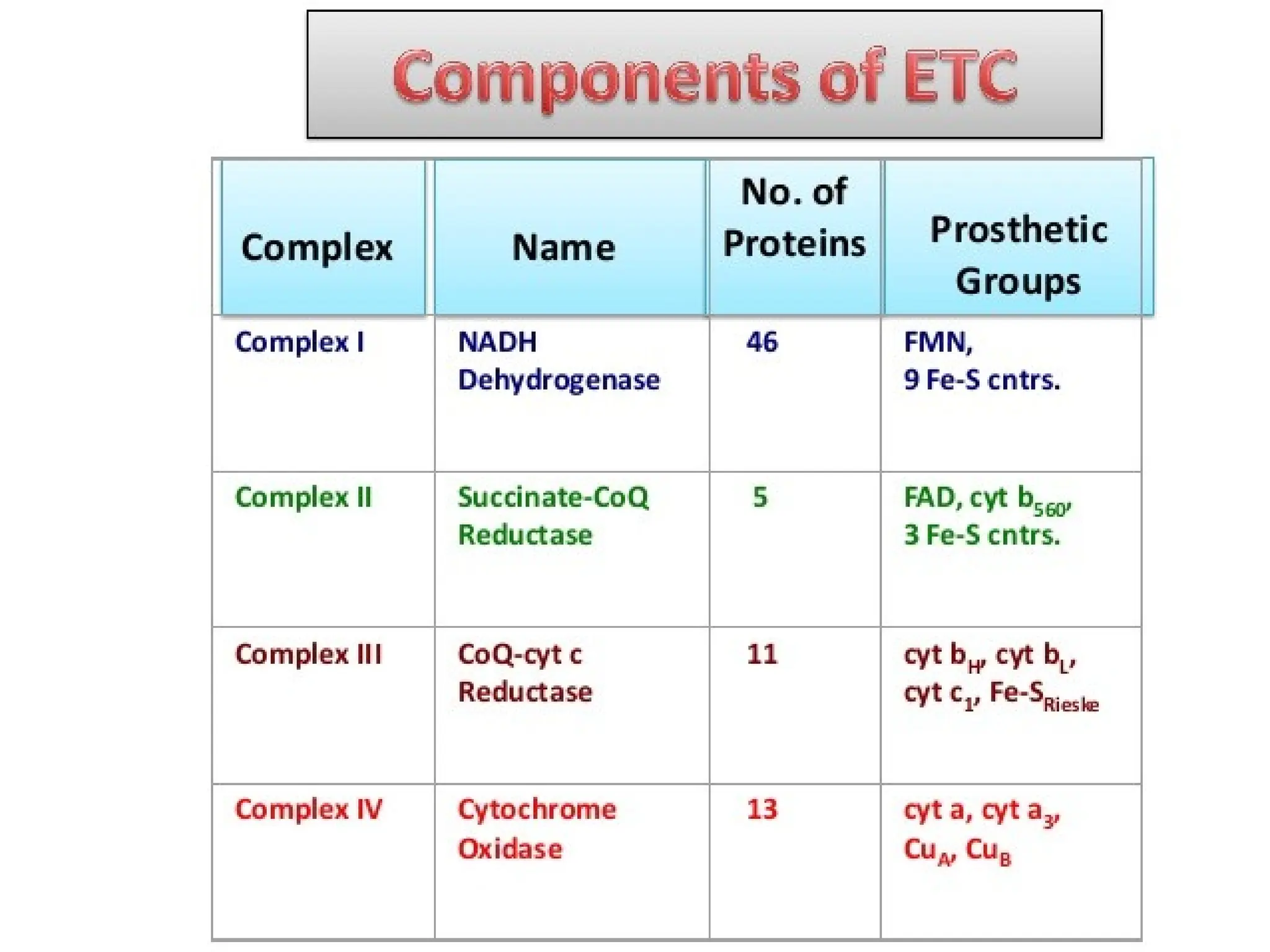
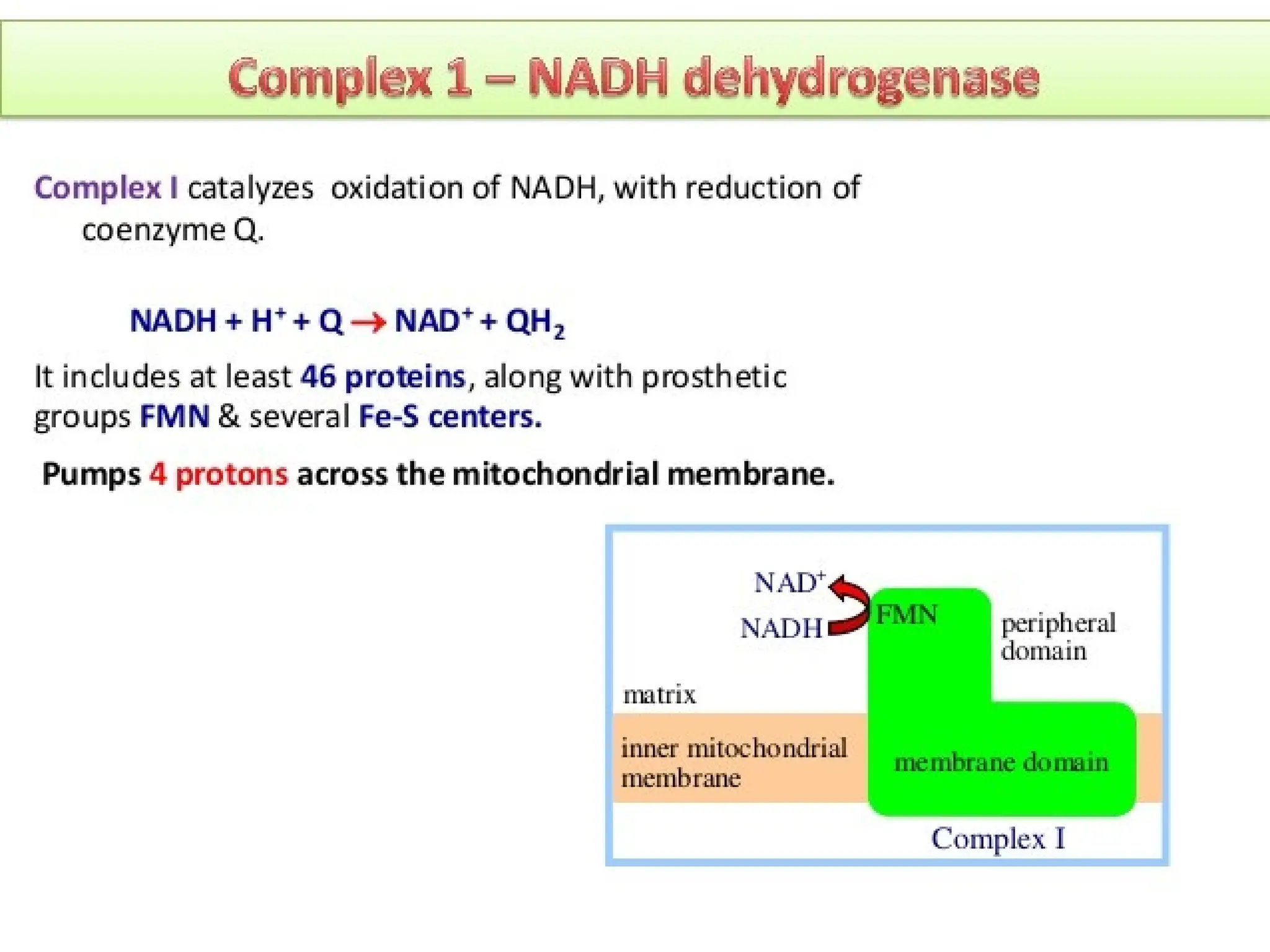
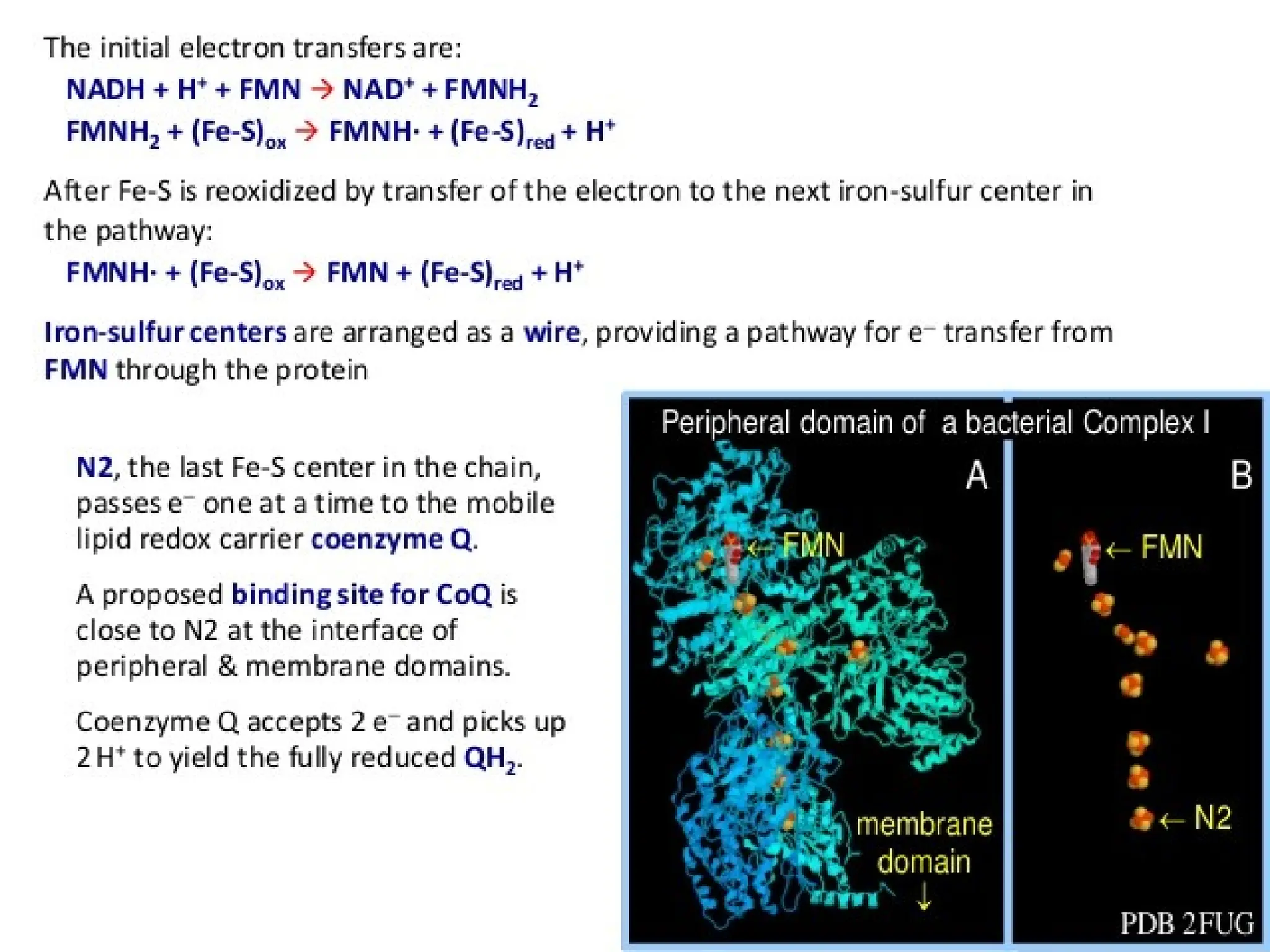
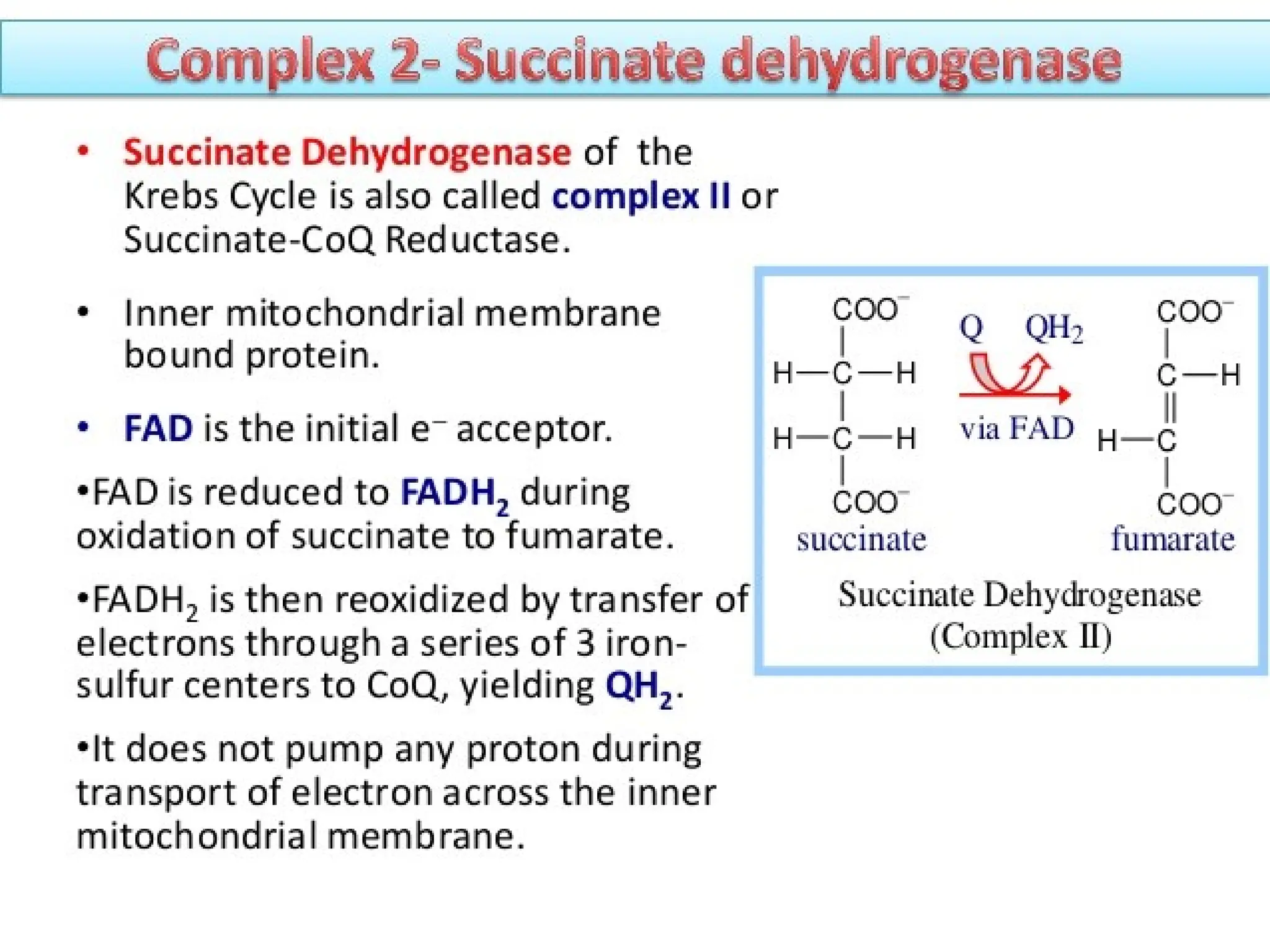
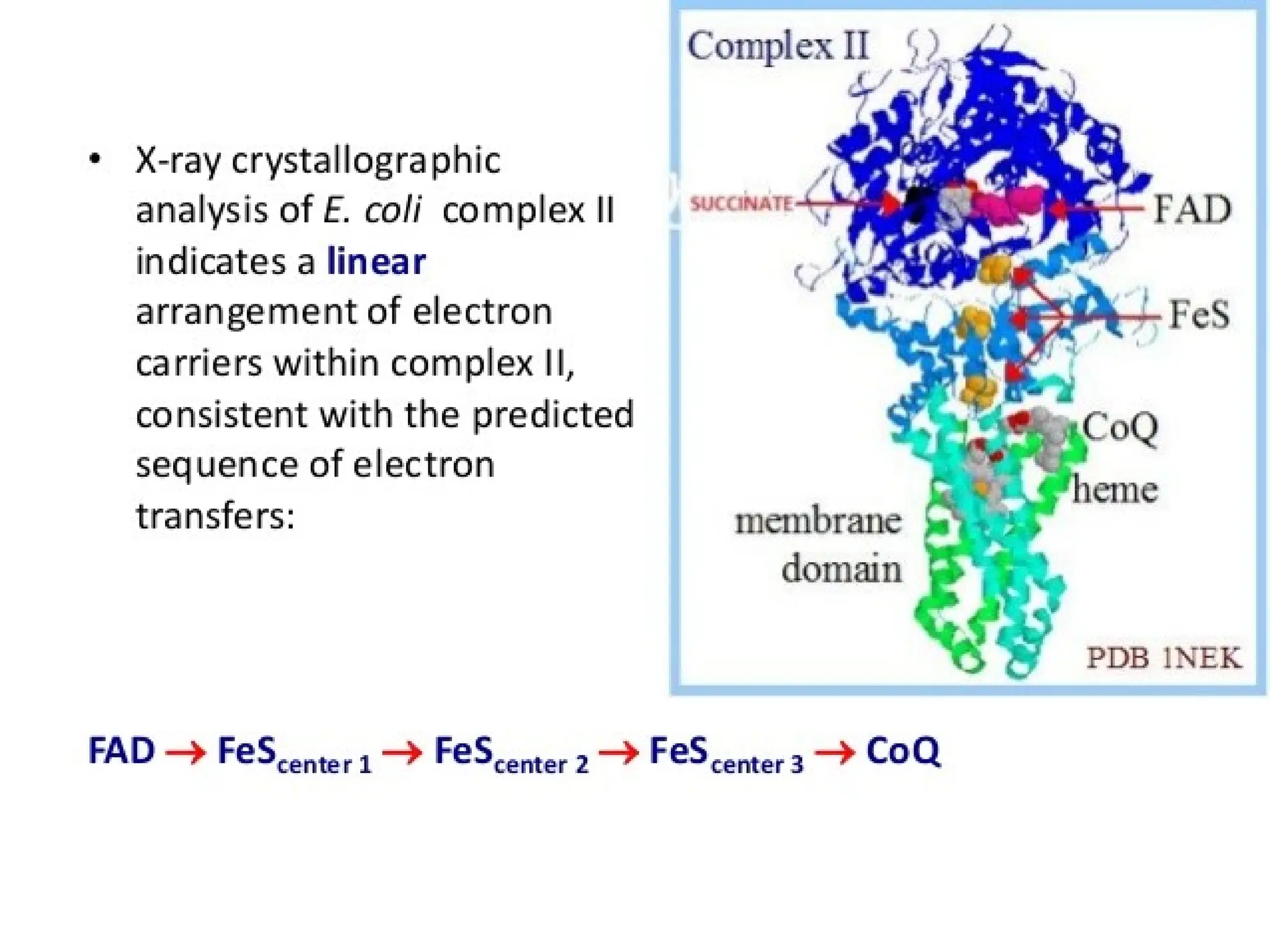
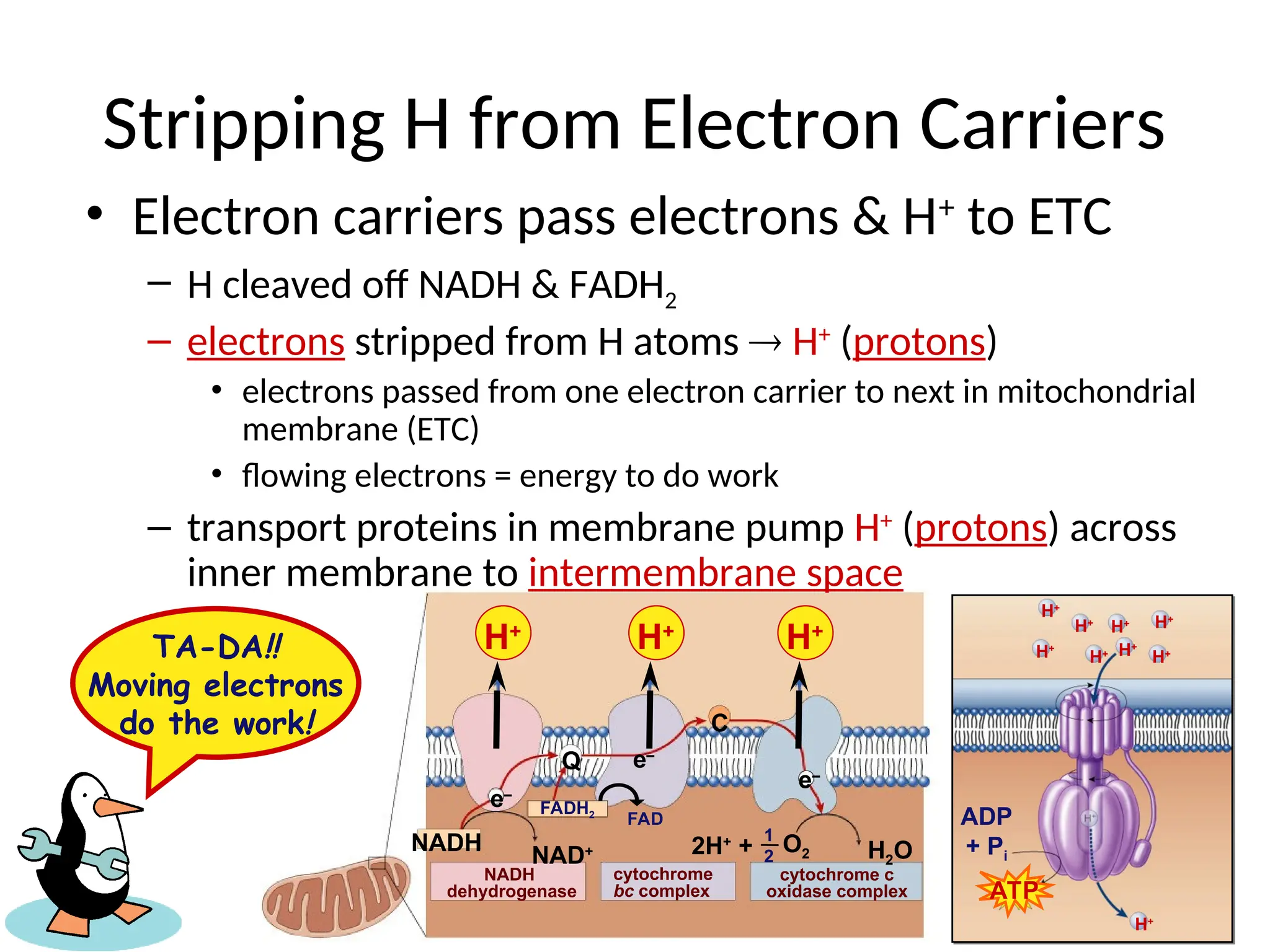
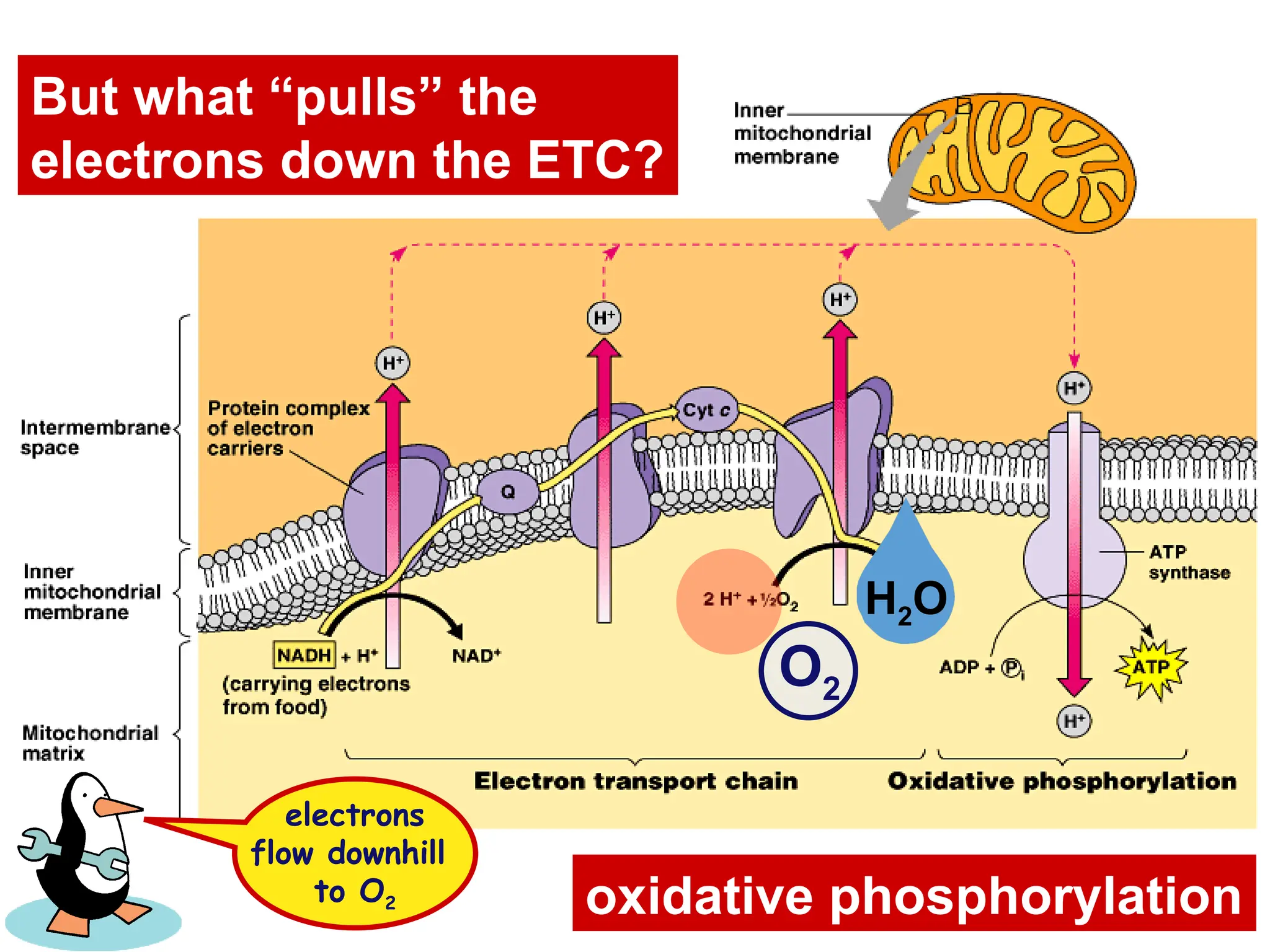
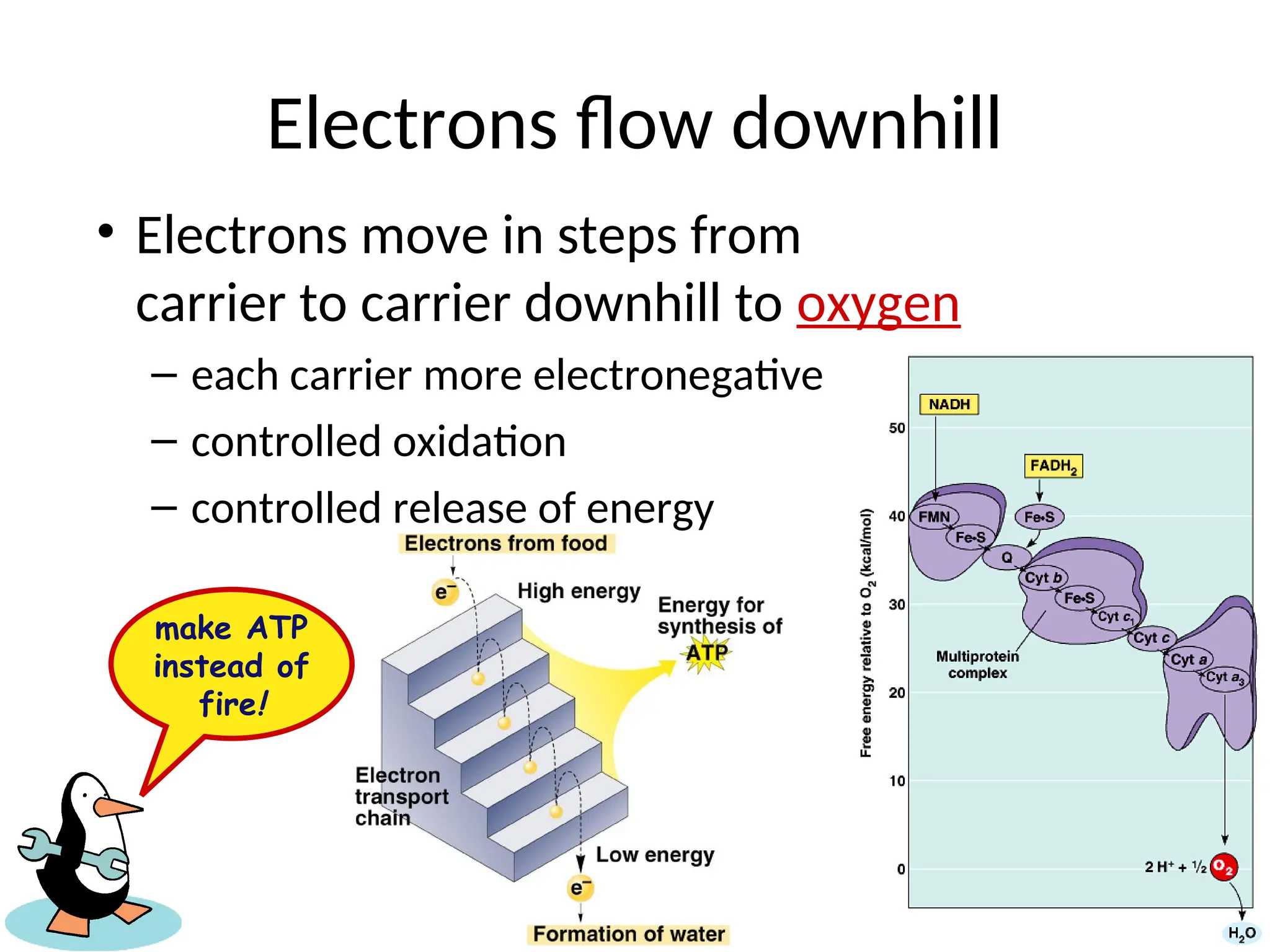
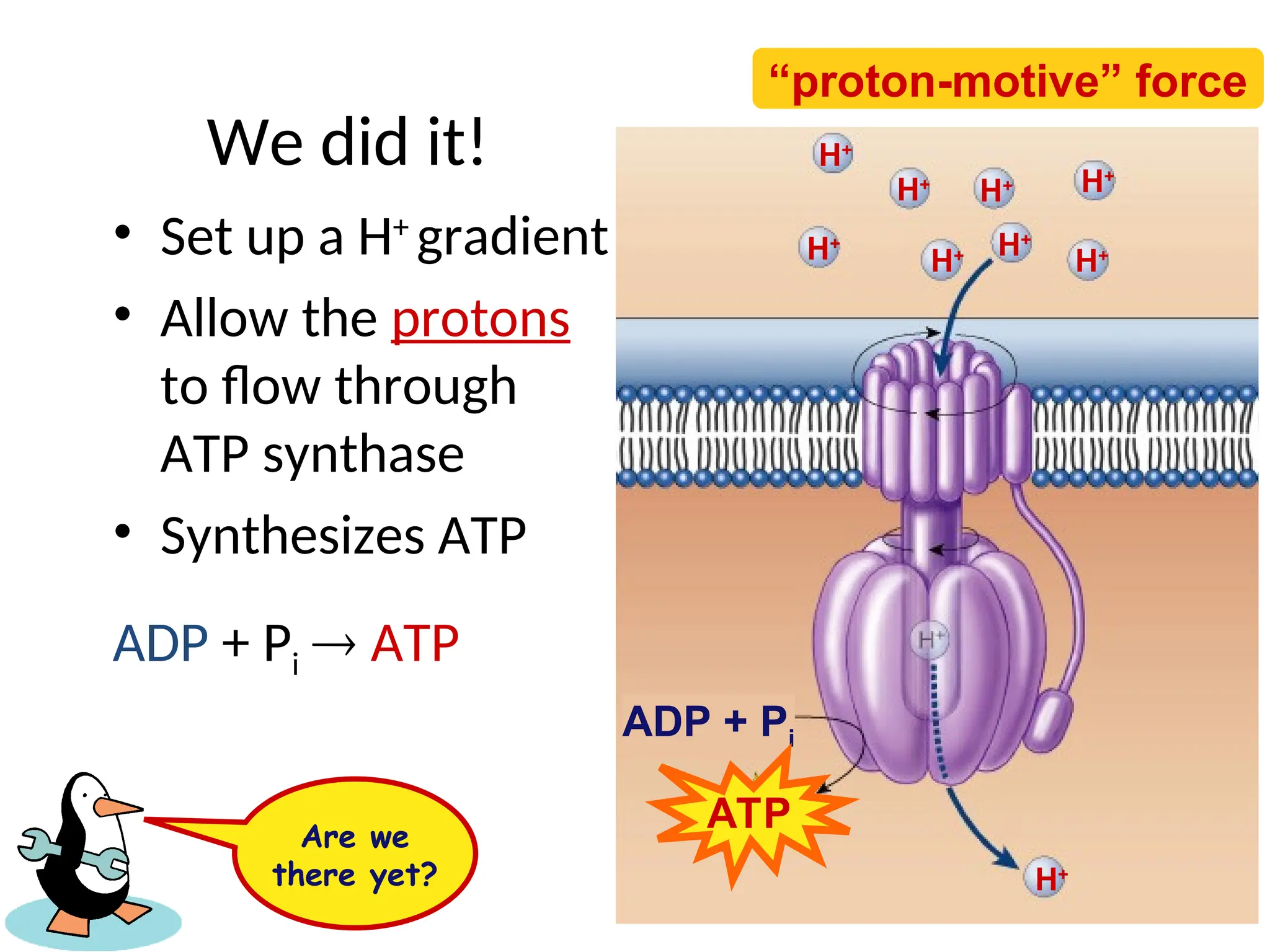
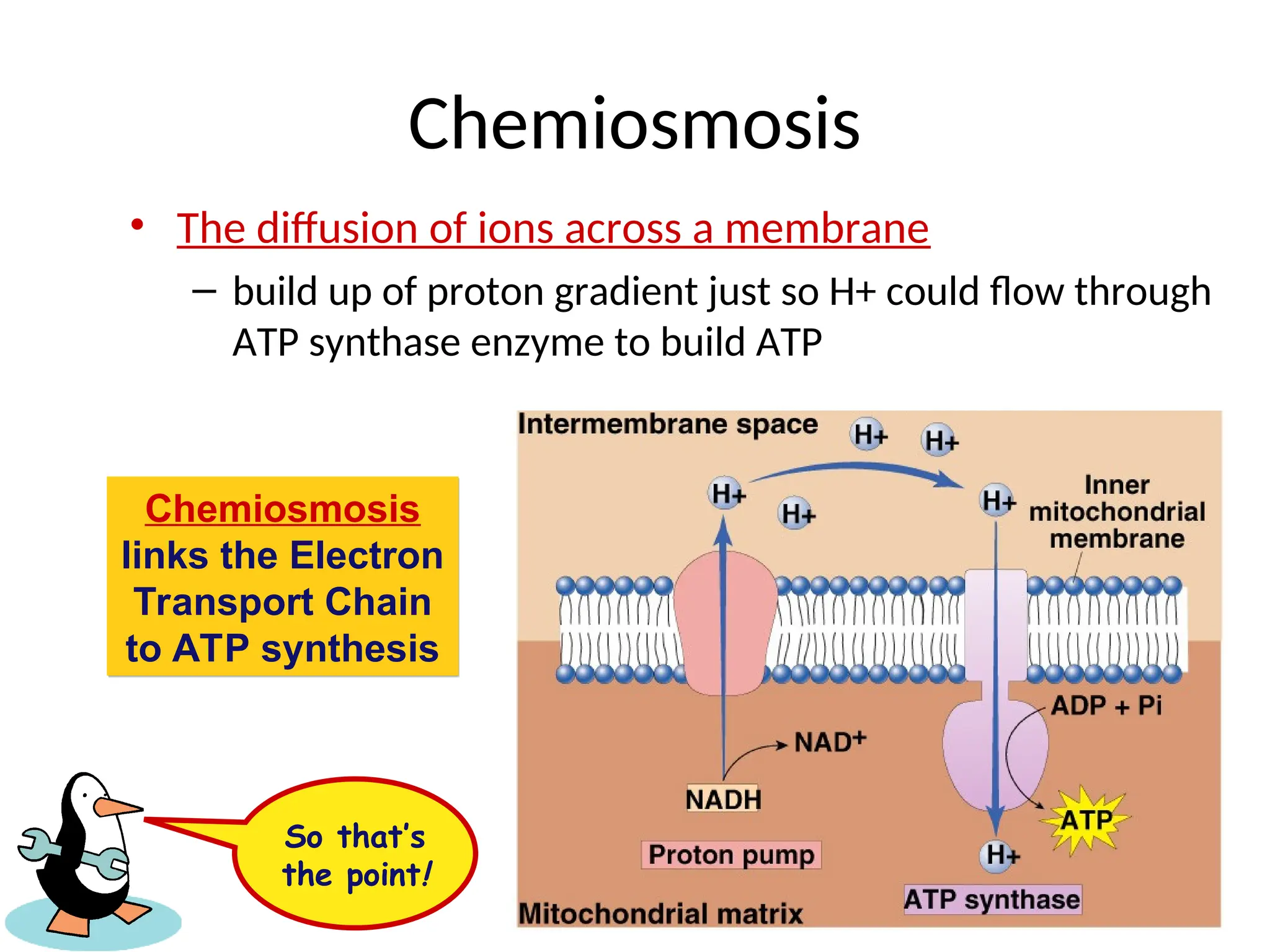
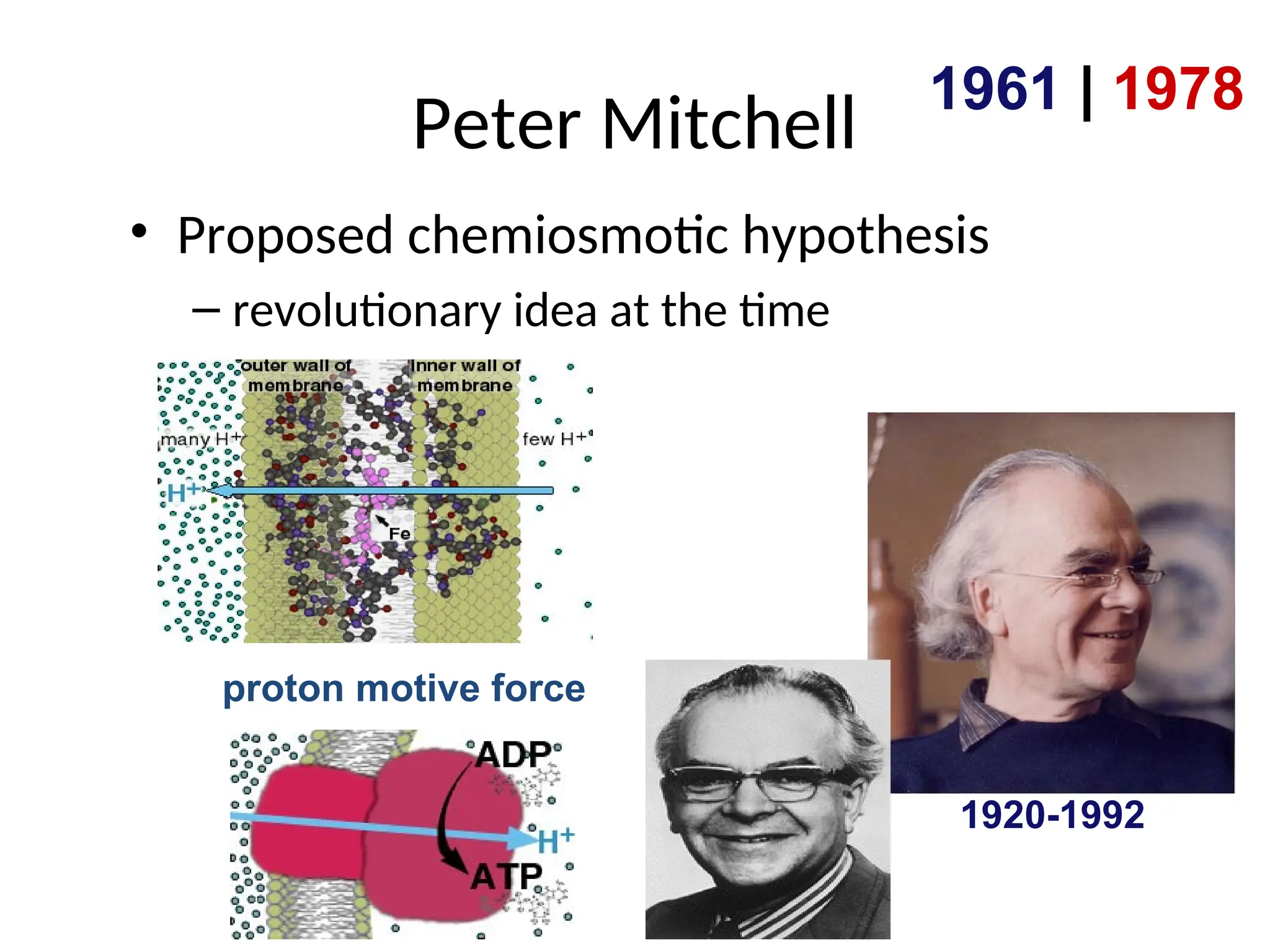
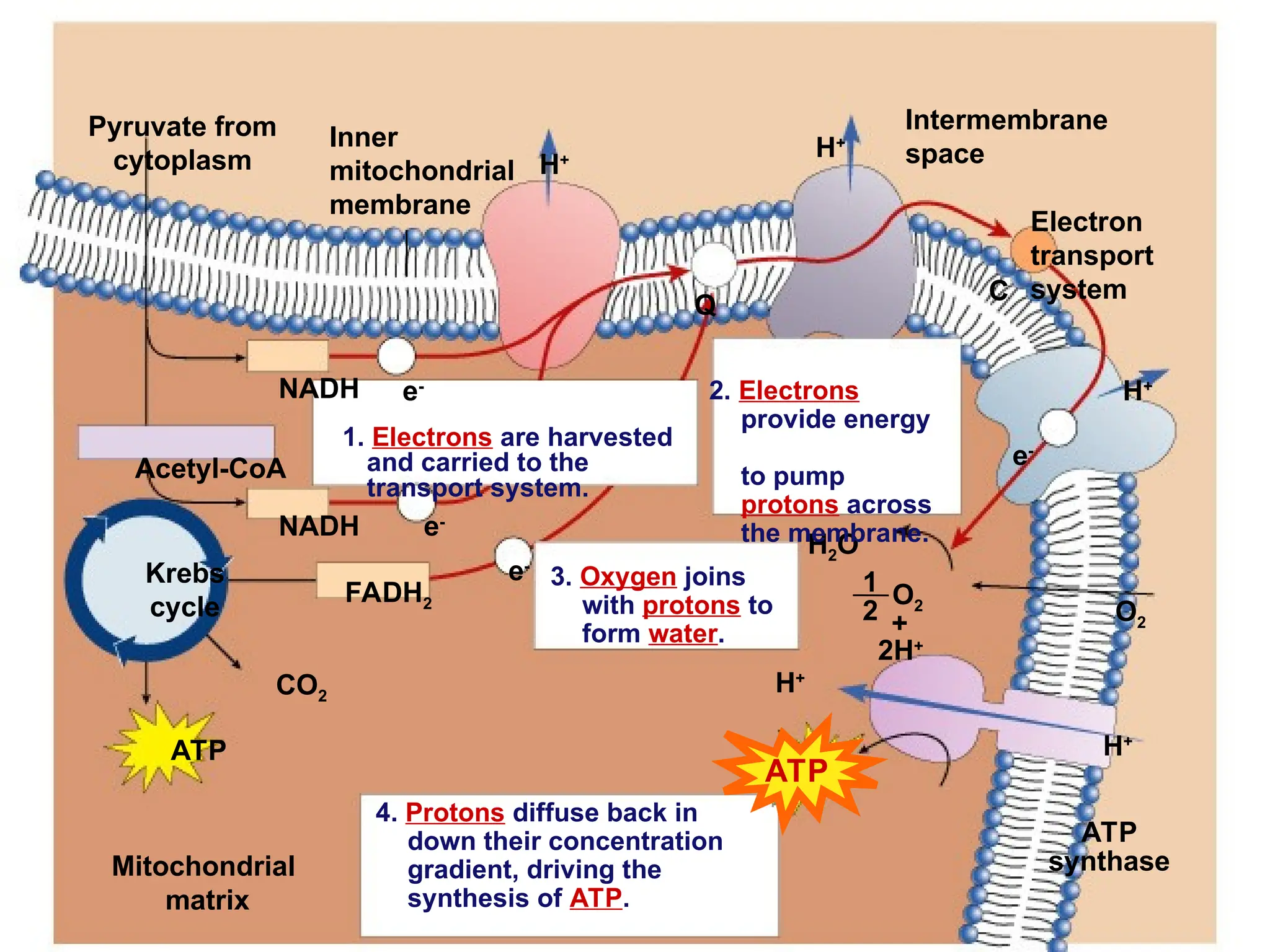
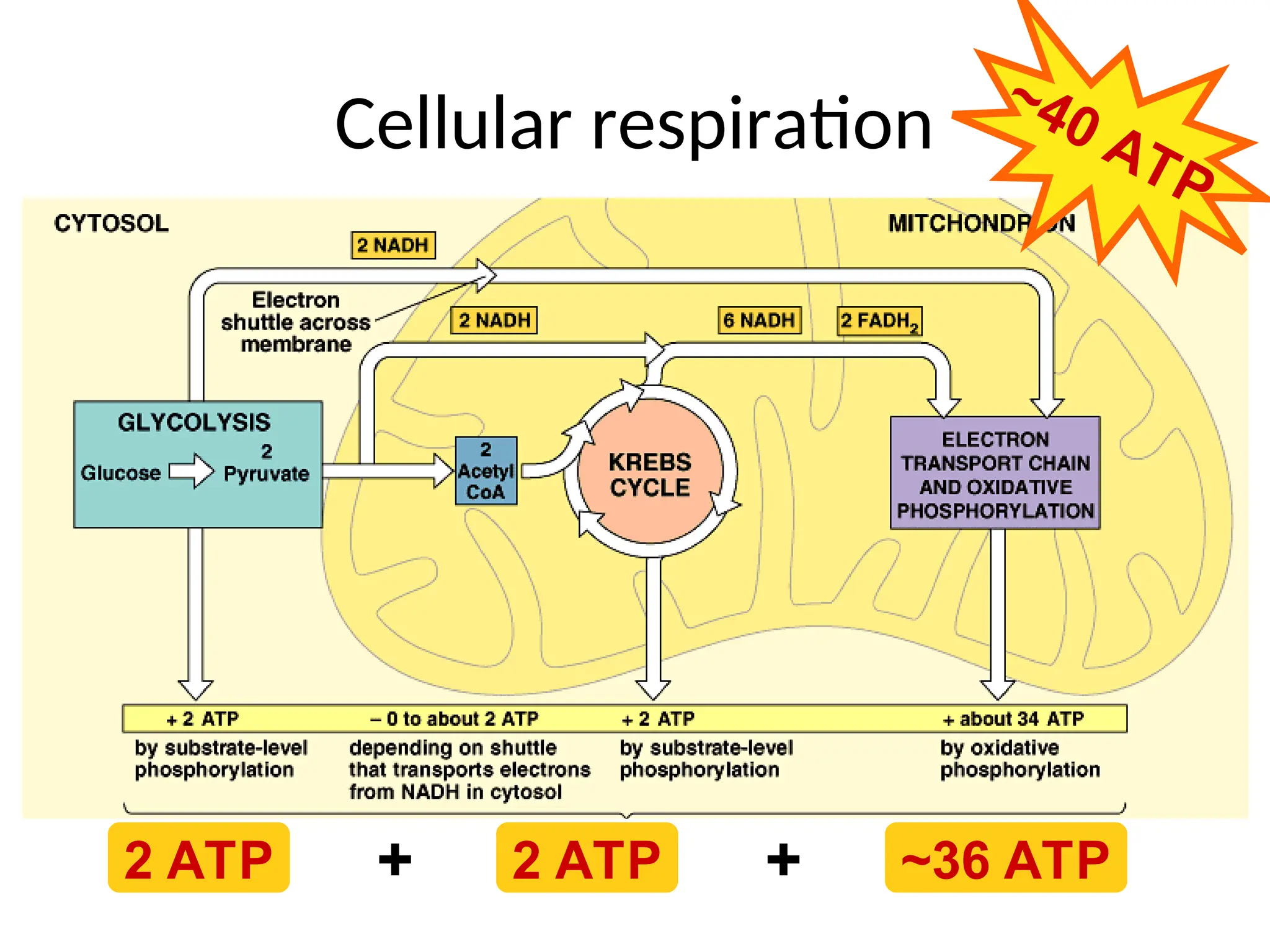
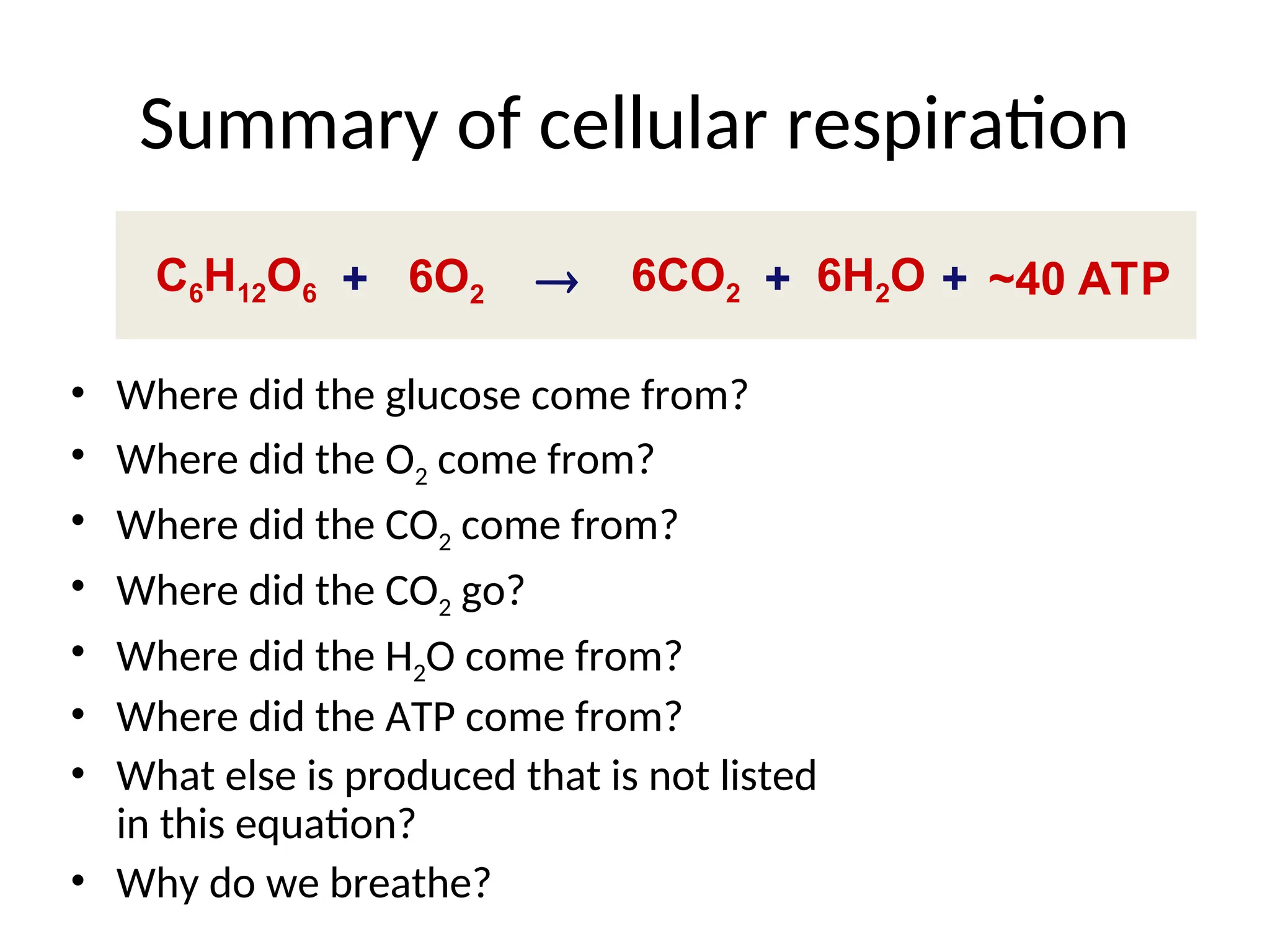
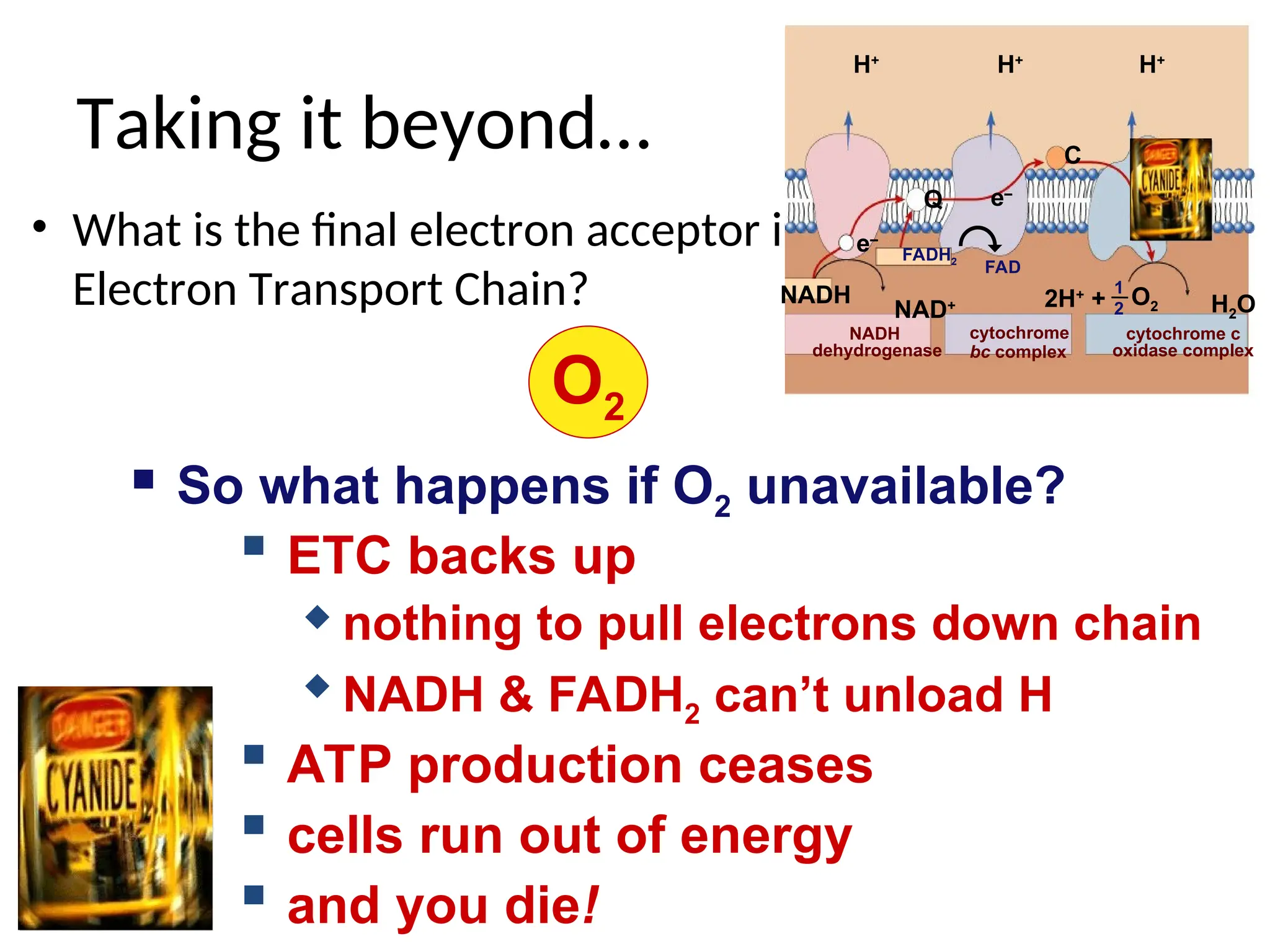
The document explains cellular respiration and the electron transport chain, highlighting that the primary purpose is to generate ATP efficiently, yielding approximately 36 ATP molecules from one glucose molecule during aerobic respiration. It describes the process of electron transport within the inner mitochondrial membrane, the creation of a proton gradient, and the role of oxygen as the final electron acceptor. Additionally, it emphasizes the importance of the chemiosmotic hypothesis proposed by Peter Mitchell in linking electron transport to ATP synthesis.