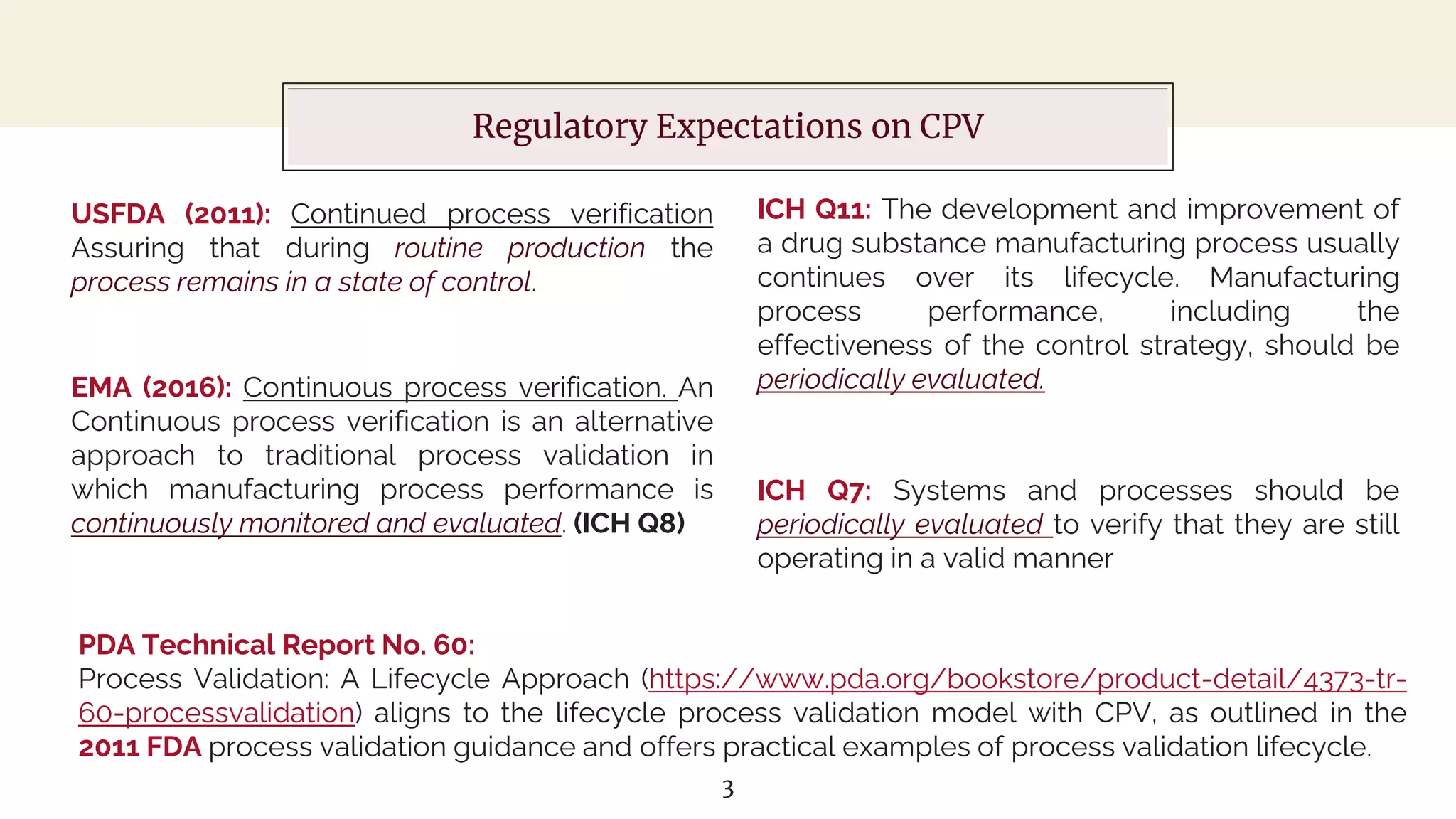
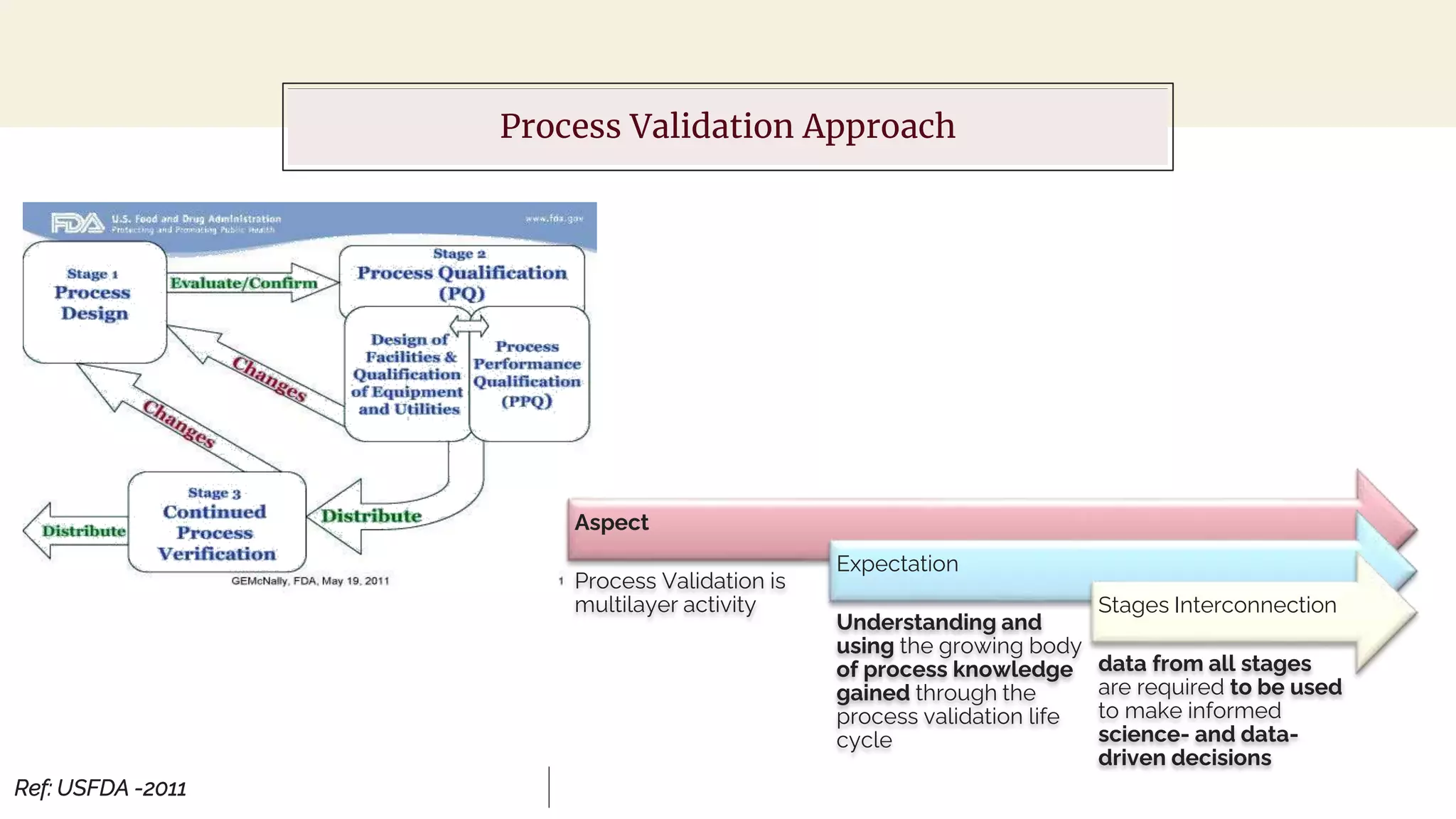
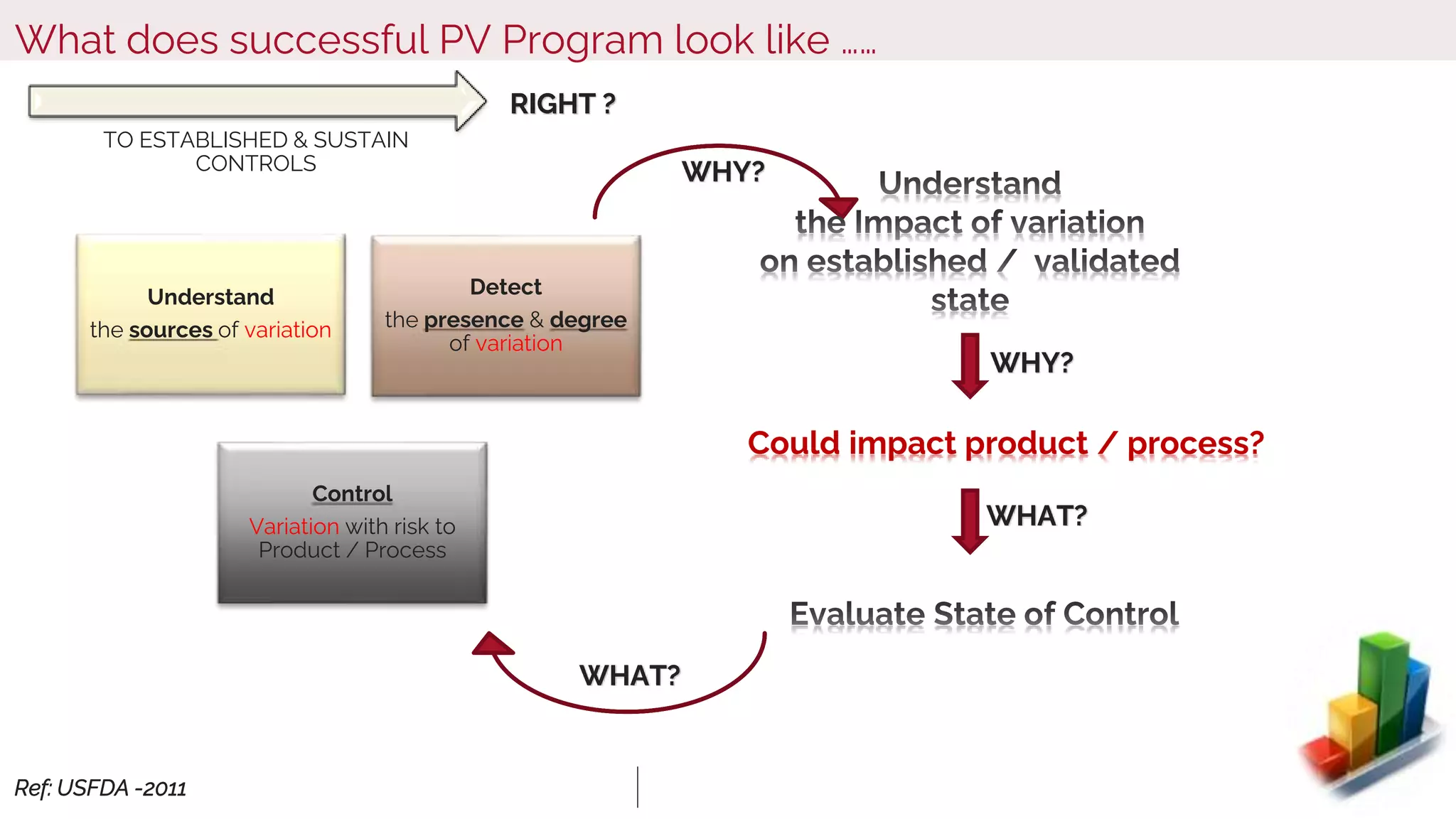
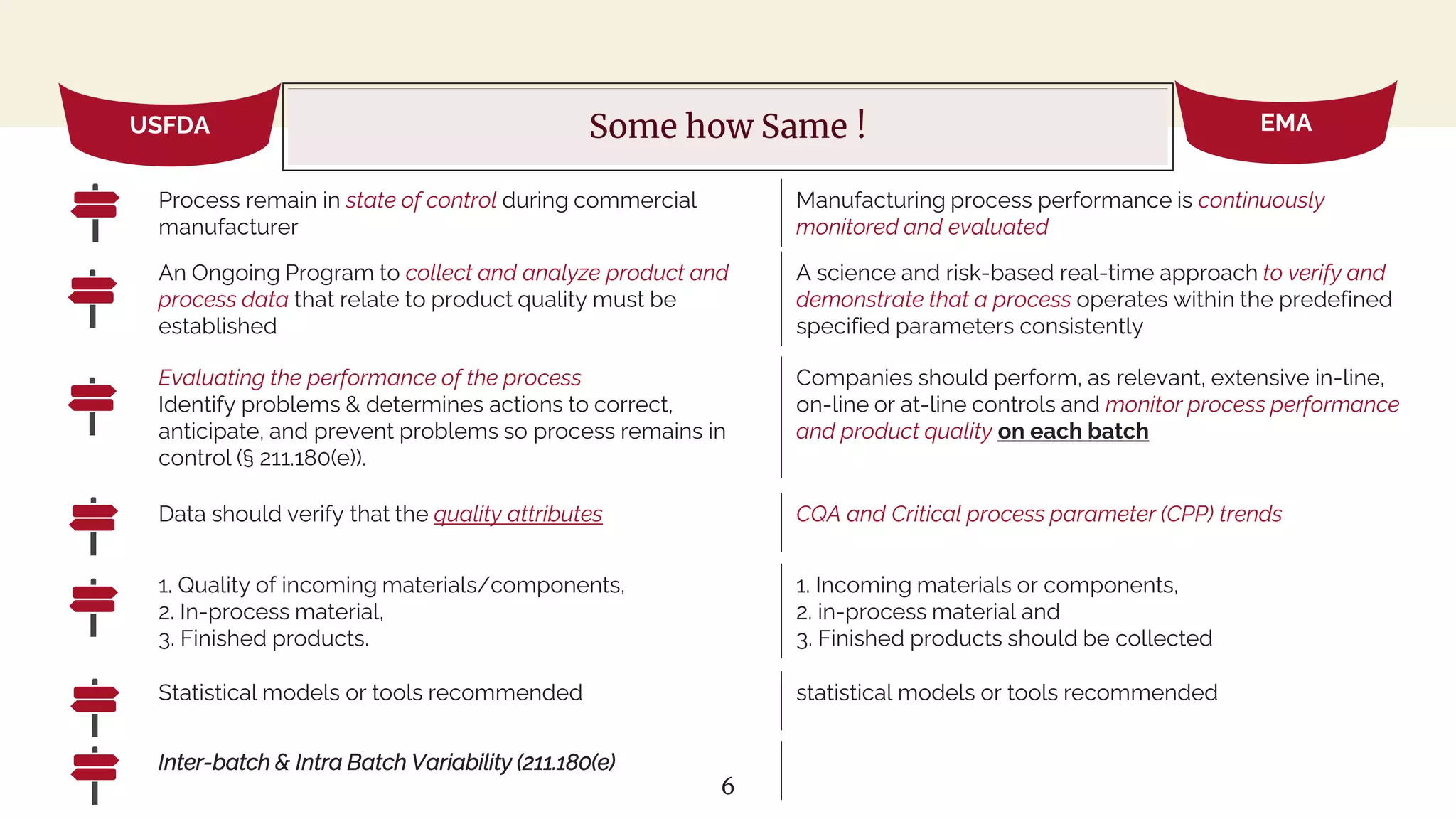
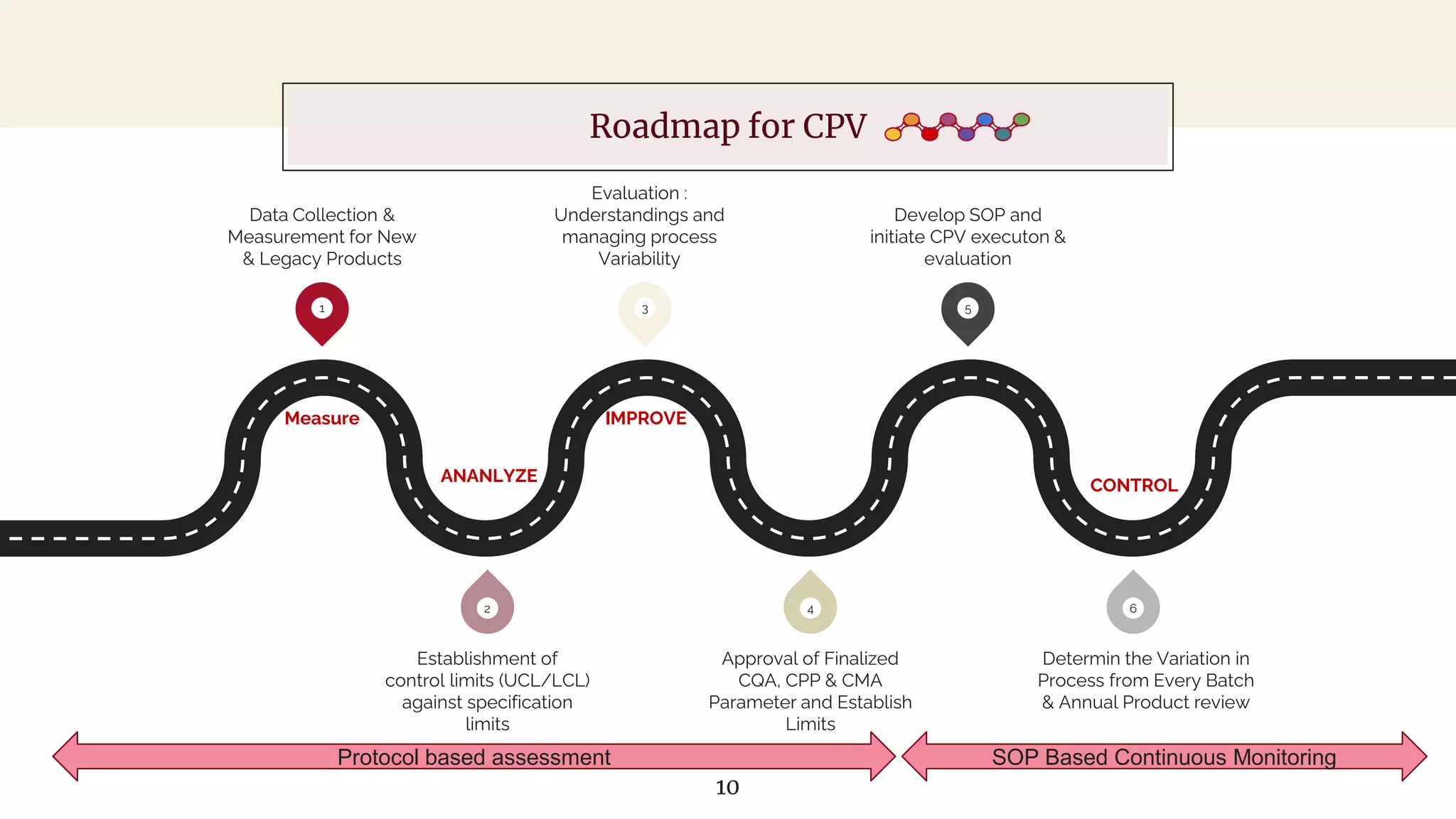
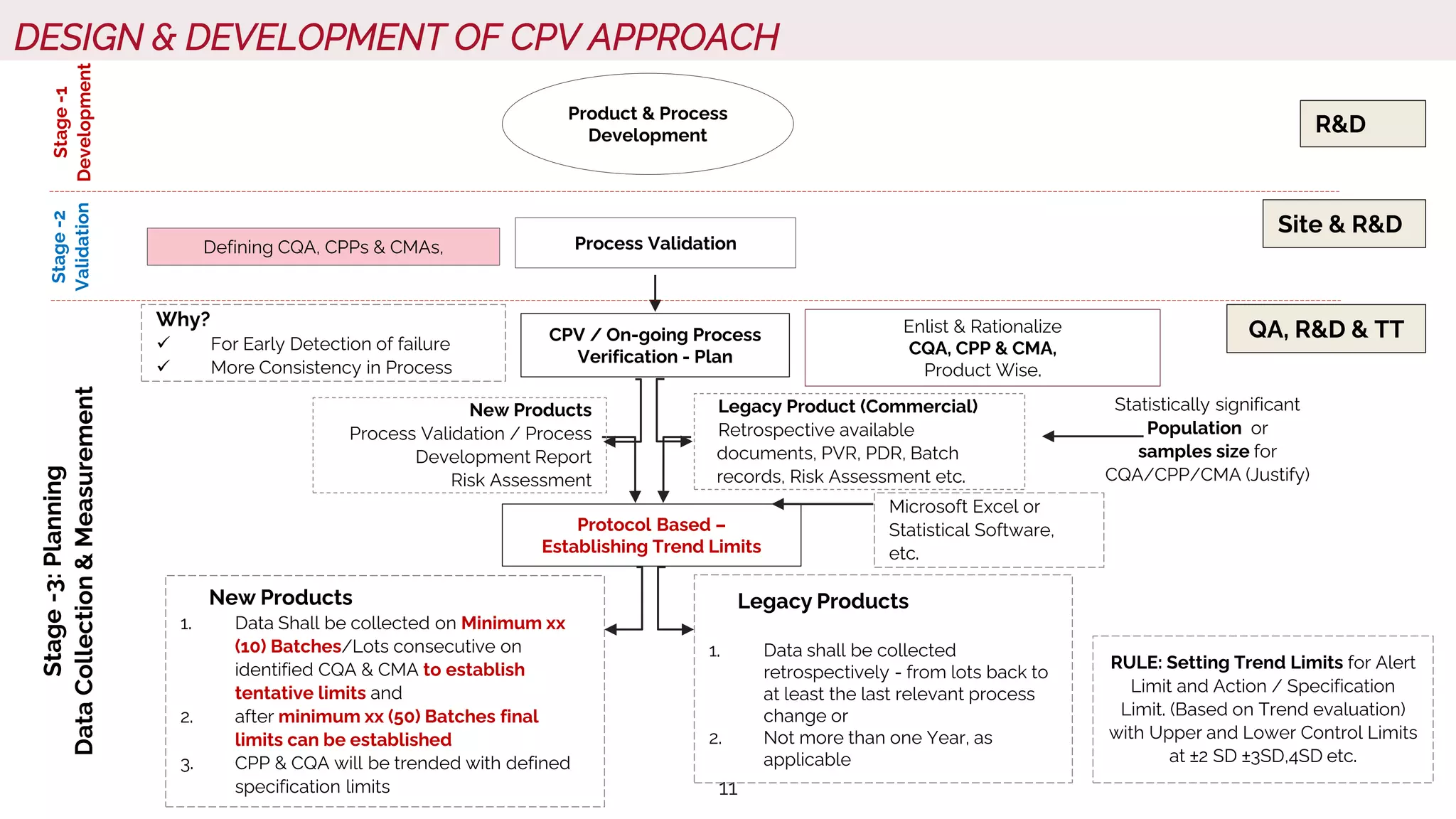
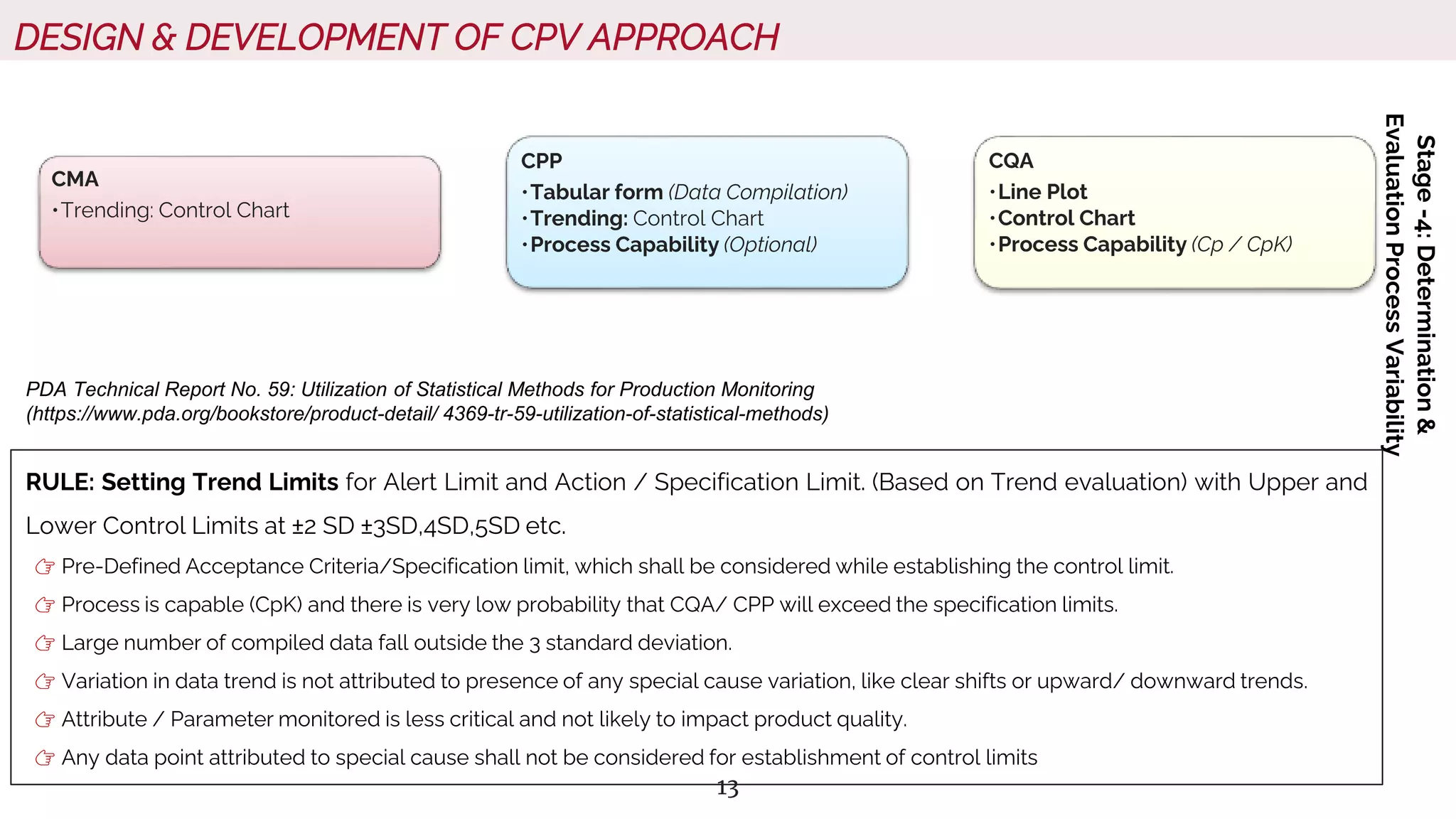
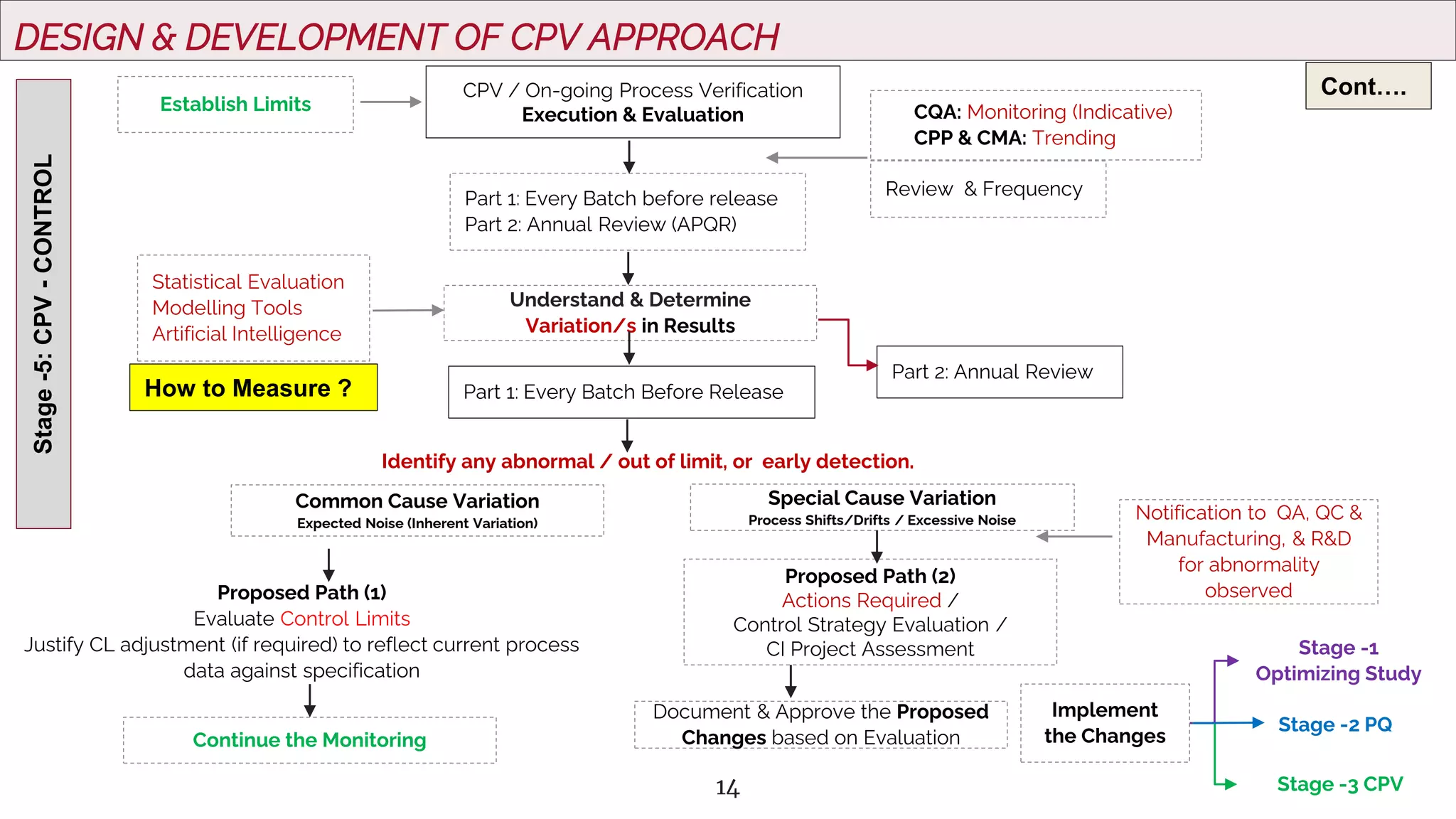
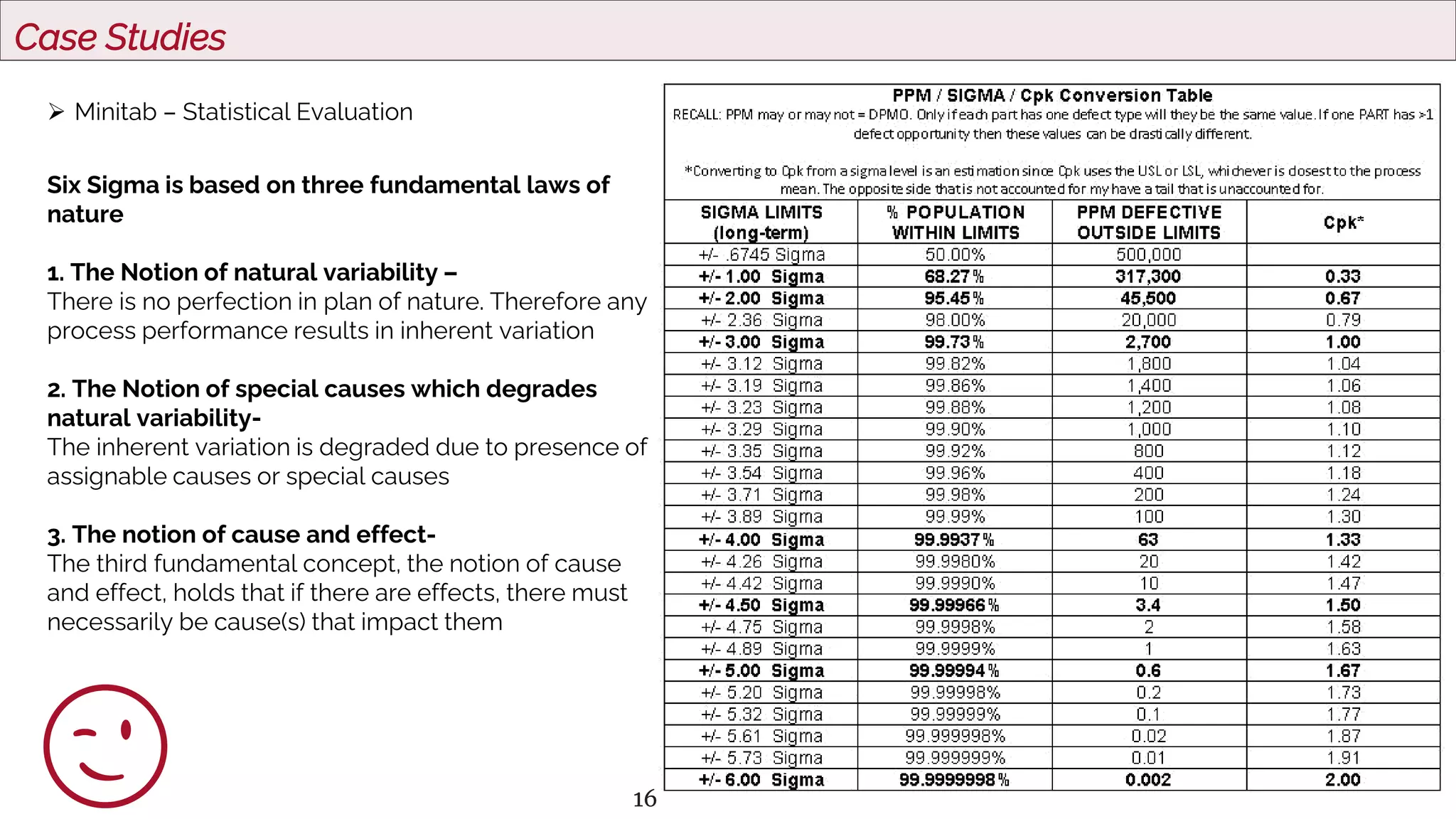
The document outlines regulatory expectations and design approaches for Continuous Process Verification (CPV), emphasizing the need for continuous monitoring and evaluation of manufacturing processes to ensure quality control throughout a drug substance's lifecycle. It discusses strategies for detecting variations in process performance, implementing risk-based assessments, and utilizing data analytics, including AI applications, to drive continuous improvement. The guidance also highlights challenges in adopting CPV and the collaboration between industry organizations and regulatory agencies to develop applicable processes and standards.