The Food and Drug Administration (FDA) is organized into 8 centers that regulate specific products and conduct research. The centers are:
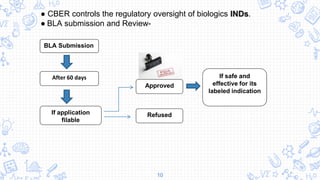
1. The Center for Biologics Evaluation and Research regulates vaccines, blood, and gene therapies.
2. The Center for Devices and Radiological Health oversees medical devices and radiation-emitting products.
3. The Center for Drug Evaluation and Research regulates prescription and over-the-counter drugs.
4. The Center for Food Safety and Applied Nutrition regulates food, dietary supplements, bottled water and cosmetics.
5. The Center for Tobacco Products regulates cigarettes and smokeless tobacco.
6. The Center for Veterinary Medicine regulates