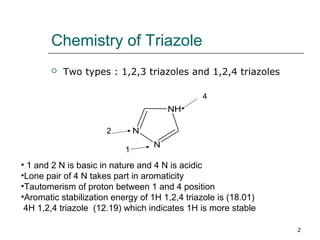
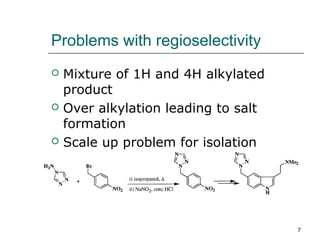
This document discusses regioselective approaches for alkylating 1H-1,2,4 triazole. It describes that 1H-1,2,4 triazole undergoes SN2 alkylation at the 1 position. The main challenges are obtaining single regioisomers and avoiding overalkylation. Weakly nucleophilic bases like DBU and using good leaving groups like tosylate improve regioselectivity and yield. Key methods include aminating the 4 position before alkylation or using DBU as the catalyst. Overall, DBU-catalyzed alkylation of 1H-1,2,4 triazole with tosylate as the living group provides high yields of the desired 1






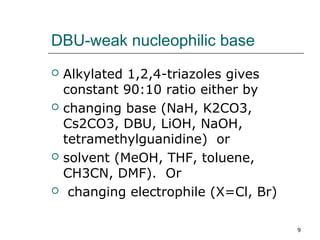
![•Regioselectivity of reaction is increased by using weak nucleophilic base
1,8 diazabicyclo[5.4.0]undec-7-ene (DBU).
•DBU (1.2 equiv.), 4-nitrobenzyl chloride (1.0 equiv.), triazole (1.1 equiv.) and
THF whereby a 93% yield of adducts in a 90:10 ratio was obtained
•successive aqueous washes increased the isomer ratio to 98:2.
•DBU is recycled and can be recovered
•DBU is water soluble
•It forms complex with HX as DBU.HX which can be seprated simply by filtration
•Purification of the isomeric mixtures of triazoles was achieved by distillation,
or silica gel chromatography or by recrystalization
•For scalling up the reaction on large scale distillation method is preferable as pure
1-alkyl-1,2,4-triazoles were obtained (>99:1) without any attempt at fractionation.
10](https://image.slidesharecdn.com/ph0057-130315120740-phpapp01/85/Regioselective-1H-1-2-4-Triazole-alkylation-10-320.jpg)


![Role of living group
+
Tosylium used here in replacement of halide group is a
good living group in presence of K2CO3 as base and
Bu4N+ •Br- as catalyst using DMF as solvent with 95%
yield [6]. To further increase the yield use of DBU as
catalyst can be done
13](https://image.slidesharecdn.com/ph0057-130315120740-phpapp01/85/Regioselective-1H-1-2-4-Triazole-alkylation-13-320.jpg)

![Refrences
[1] POTTS . K. T.; THE CHEMISTRY OF 1,2,4-
TRIAZOLES;Tetrahedron,1960
[2] ATKINSON. M. R.; POLYA. J. B.; J.Org.
Chem., 1952, 3418.
[3] Katritzky et. al.; Hetrocyclic aromatic
chemistry
[4] Balaban.A.T.; Oniciu.C.D.; Esperion
Therapeutics;2003;2777
[5] Paul G. B., Ian F. C., Cameron J. C.;
Tetrahedron Letters 41 (2000) 1297–1301
[6] Aouine, Younas; Faraj, Hassane;Molecules,16;
2011; 3380-3390
[7] Chen, Wei; Chen, Chen;
Journal;33;7;2011;603-606
15](https://image.slidesharecdn.com/ph0057-130315120740-phpapp01/85/Regioselective-1H-1-2-4-Triazole-alkylation-15-320.jpg)
