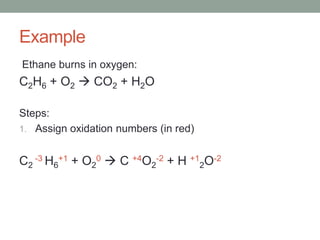
Redox reactions involve the transfer of electrons from one reactant to another. The substance that loses electrons is oxidized and acts as the reducing agent, while the substance that gains electrons is reduced and acts as the oxidizing agent. This is demonstrated through an example of the combustion of ethane, where carbon is oxidized and loses electrons, acting as the reducing agent, while oxygen is reduced and gains electrons, acting as the oxidizing agent. A practice problem on the reaction between chlorine and potassium iodide is then worked through using the same steps to determine oxidation, reduction, and the reducing and oxidizing agents.