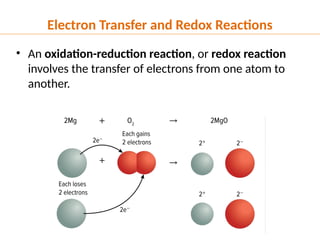
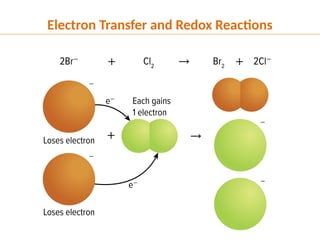
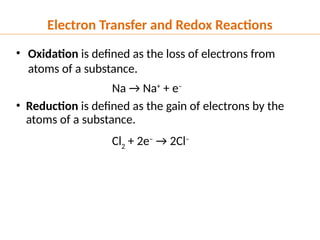
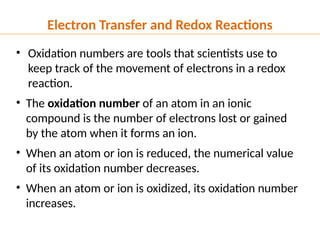
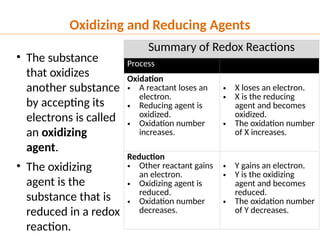
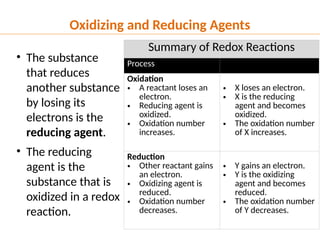
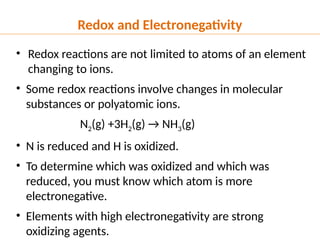
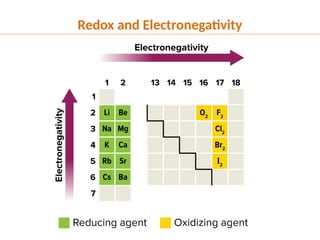
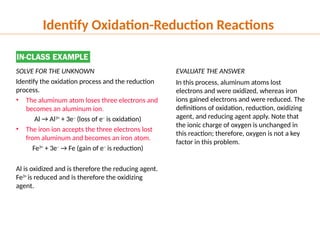
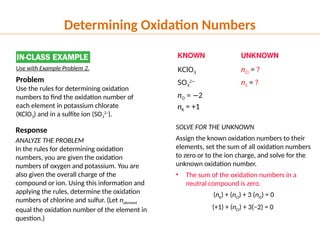
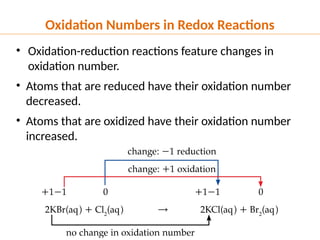
The document discusses oxidation and reduction (redox) reactions, defining oxidation as the loss of electrons and reduction as the gain of electrons, and introduces key vocabulary such as oxidizing agents and reducing agents. It explains the identification of oxidation numbers and the roles of these agents in electron transfer processes within redox reactions. Additionally, it provides examples and problems for better understanding of how to determine what is oxidized or reduced in a reaction.