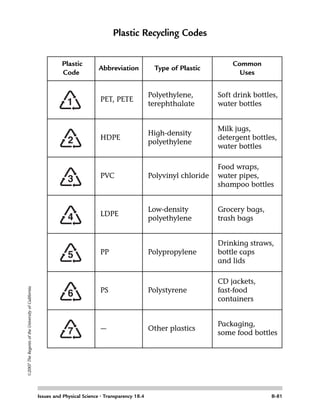
Students determine the chemical and physical properties of four common plastics - polypropylene, polyvinyl chloride, high-density polyethylene, and polystyrene. They test samples of each plastic using various liquids and heat to observe properties like flexibility, hardness, and density. Students then test an unknown "mystery" plastic sample to determine its properties and identify which of the four plastics it is based on the evidence from the tests. The key is relating the plastics' observed properties to possible uses based on how those properties affect functionality.