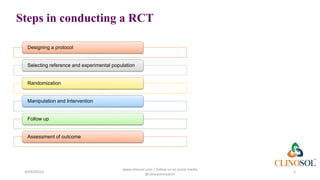
The document discusses randomized clinical trials (RCTs) as essential tools in clinical research, providing high-quality evidence for healthcare decision-making and considered the gold standard for evaluating medical interventions. It outlines the steps involved in conducting RCTs, including protocol design, population selection, randomization, intervention, follow-up, and assessment of outcomes. The conclusion emphasizes the importance of randomization in minimizing bias and establishing causality.