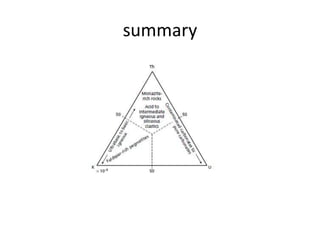
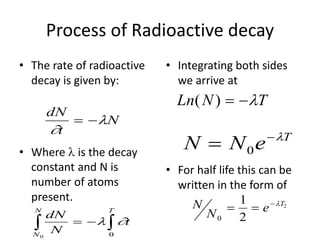
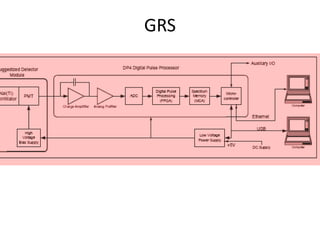
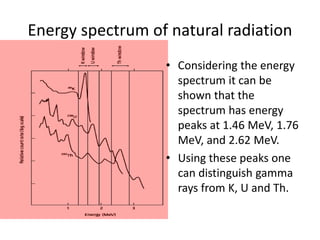
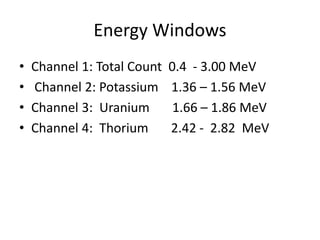
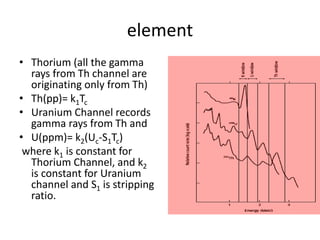
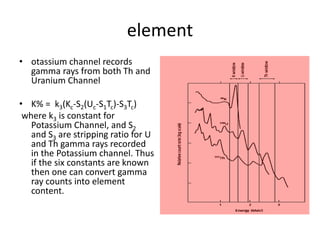
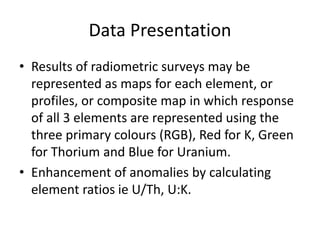
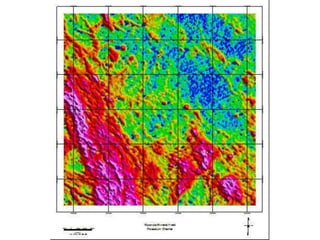
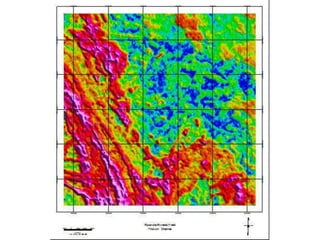
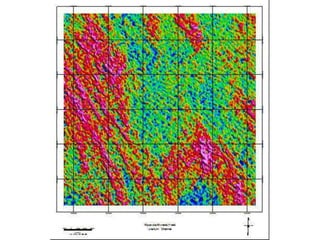
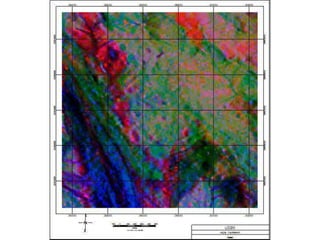
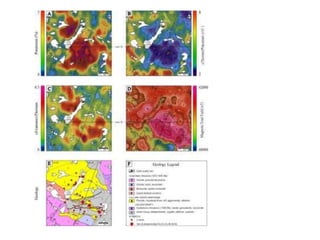
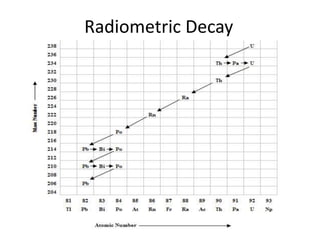
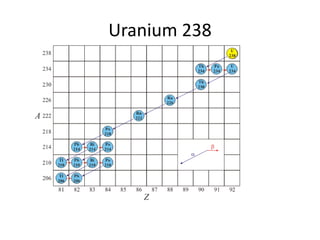
Radiometric methods involve detecting gamma rays from geological environments through various approaches, relying on natural radioactivity first discovered by Henri Becquerel. Key elements examined include uranium, thorium, and potassium, which are used for dating rocks and mineral prospecting due to their natural occurrence and properties. The document also outlines the types of instruments used for detection and the importance of calibration for accurate radiometric surveys.








![Potassium Minerals
• Mineral
• (i) Orthoclase and microcline feldspars [KAlSi3O8]
• (ii) Muscovite [H2KAl(SiO4)3]
• (iii) Alunite [K2Al6(OH)12SiO4]
• (iv) Sylvite, carnallite [KCl, MgCl2.6H2O]
• Occurrence
• (i) Main constituents in acid igneous rocks and pegmatites
• (ii) Main constituents in acid igneous rocks and pegmatites
• (iii) Alteration in acid volcanics
• (iv) Saline deposits in sediments](https://image.slidesharecdn.com/radiometricmethods-240618112358-5818a2c8/85/Radiometric-Methods-isotops-Alpha-and-Beta-Particles-14-320.jpg)
![Thorium Minerals
(i) Monazite [ThO2 + rare earth phosphate]
(ii) Thorianite [(Th,U)O2]
(iii) Thorite, uranothorite [ThSiO4 + U]
• Occurrence
• (i) Granites, pegmatites, gneiss
• (ii), (iii) Granites, pegmatites, placers](https://image.slidesharecdn.com/radiometricmethods-240618112358-5818a2c8/85/Radiometric-Methods-isotops-Alpha-and-Beta-Particles-15-320.jpg)
![Uranium Minerals
• (i) Uraninite [oxide of U, Pb, Ra +Th, rare earths]
• (ii) Carnotite [K2O.2UO3.V2O5.2H2O]
• (iii) Gummite [uraninite alteration]
• Occurrence
• (i) Granites, pegmatites and with vein deposits of
Ag, Pb, Cu, etc.
• (ii) Sandstones
• (iii) Associated with uraninite](https://image.slidesharecdn.com/radiometricmethods-240618112358-5818a2c8/85/Radiometric-Methods-isotops-Alpha-and-Beta-Particles-16-320.jpg)