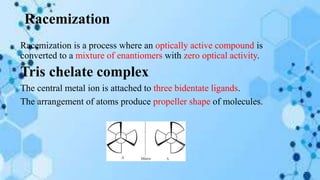
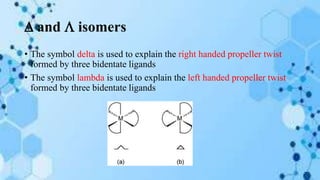
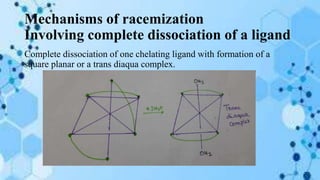
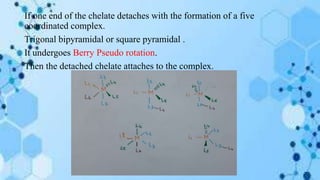
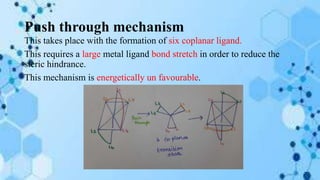
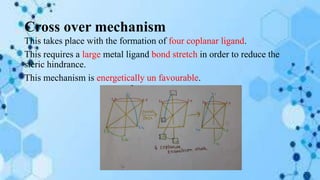
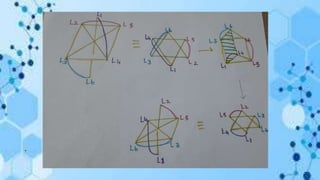
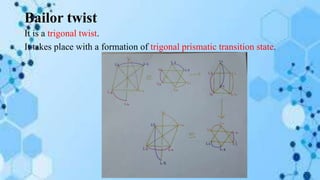
The document discusses racemization, a process converting an optically active compound into a mixture of enantiomers, and mechanisms involved, including complete dissociation and bond rupture without dissociation. Various mechanisms, such as push through, cross over, bailor twist, and ray dutt twist, are explained in terms of their conditions and energetics. Additionally, mechanisms are categorized based on whether the rate of racemization exceeds the rate of dissociation, influencing the type of twist that occurs in the ligand arrangement.