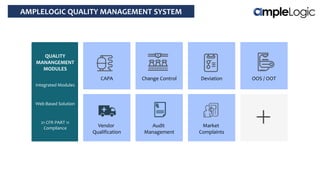
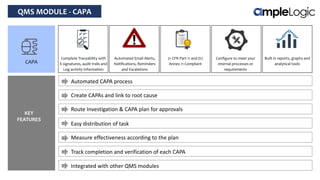
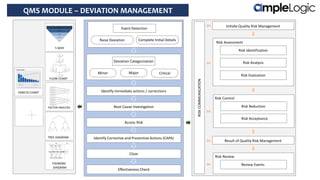
This document describes various quality management system modules provided by AmpleLogic, including CAPA, change control, deviation management, audit management, vendor qualification, and market complaints. For each module, it discusses regulatory requirements, problems with traditional manual systems, and key features of AmpleLogic's computerized solutions. The solutions provide features like electronic records, signatures and audit trails, integration between modules, and automated notifications and reports. AmpleLogic is an established quality management software provider that works with many global pharmaceutical companies.