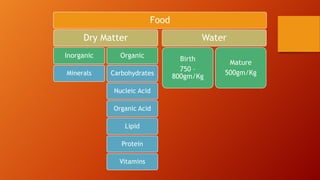
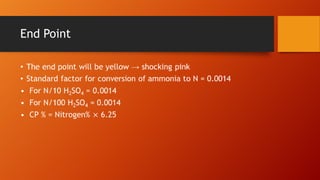
This document provides information and procedures for conducting a proximate analysis of a feed or food sample. It describes the key components that are determined in a proximate analysis including moisture, crude protein, ether extract, crude fiber, and ash. For each component, it outlines the general principle, necessary reagents, and step-by-step procedure for how to analyze a sample and calculate the percentage of that component. The overall document serves as a guide for setting up an analytical laboratory and conducting the various tests involved in a complete proximate analysis.