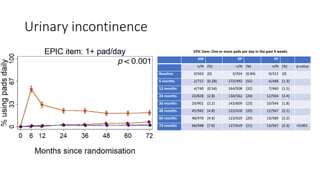
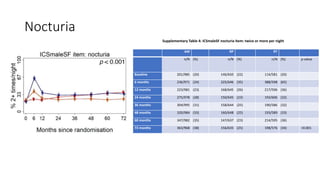
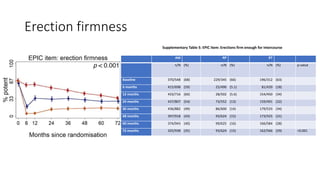
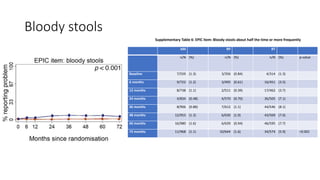
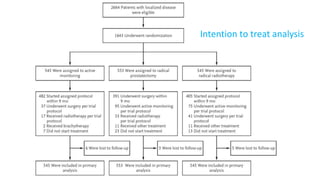
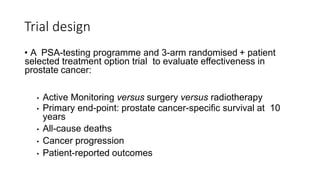
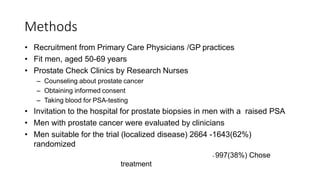
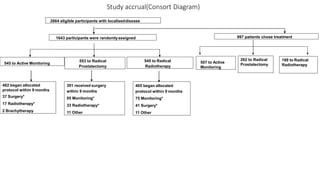
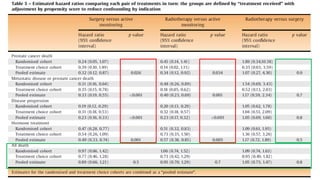
This document summarizes a journal club discussion on a clinical trial comparing active monitoring, surgery, and radiotherapy for treating clinically localized prostate cancer. The trial included over 2,600 men randomized to one of the three treatment groups or choosing their own treatment. Results found no difference in prostate cancer deaths between groups after 10 years. Exploratory analyses combining randomized and non-randomized cohorts found a lower risk of cancer death with radical treatment versus active monitoring. However, radical treatments were associated with higher rates of urinary incontinence, erectile dysfunction, and bowel issues compared to active monitoring. Limitations included potential for bias in the analyses and unknown long-term outcomes beyond 10 years.











![• Propensity scores were generated using multinomial logistic
regression, with treatment received as the three-category outcome
variable, and the following covariates: cT stage (1 or 2), Gleason grade
group (1, 2, 3, and >3 [4 and 5 combined]), log-transformed PSA, age
in years, and study center.
• Data from the two cohorts were combined, and survival curves for
prostate cancer mortality and metastasis drawn
• For PROMs data from the cohorts were combined, and all data
after treatment were compared between treatment groups](https://image.slidesharecdn.com/protecttrial-200424100510/85/Protec-t-trial-Journal-club-16-320.jpg)
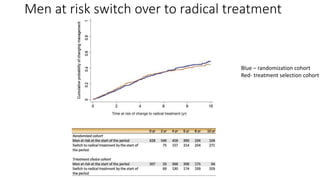
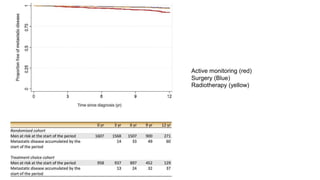
![• Most progression
• development of a high stage (T3 orT4)
• starting of ADT
• Higher in the AM group (60% [85/142] in the randomized cohort and
57% [45/79] in the treatment choice cohort).
• The presence of stage T3 or T4 did not prevent subsequent radical
treatment in some men, with 20% (22/112) undergoing RP and 35%
(39/112) undergoing RT.](https://image.slidesharecdn.com/protecttrial-200424100510/85/Protec-t-trial-Journal-club-24-320.jpg)
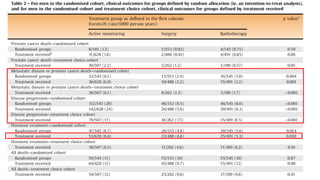
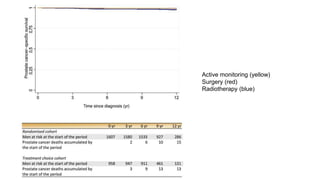
![Exploratory ancillary analyses
• Combined surgery group + RT group - compared with AM
• Reduction in prostate cancer-specific mortality with radical treatment compared with AM
• Randomised cohort (hazard ratio = 0.34; 95% [CI] 0.13, 0.94)
• Treatment choice cohort (hazard ratio = 0.27; 95% CI 0.08, 0.91)
• Pooling of these two estimates - marked decrease in prostate cancer mortality with
radical treatment (hazard ratio = 0.31; 95% CI 0.14, 0.67; p = 0.003)
• Small number of prostate cancer deaths means that the absolute reduction is modest!!](https://image.slidesharecdn.com/protecttrial-200424100510/85/Protec-t-trial-Journal-club-25-320.jpg)