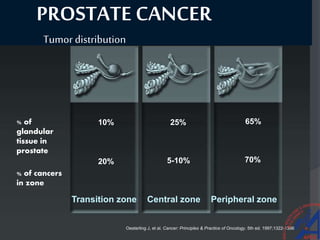
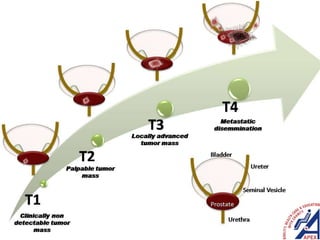
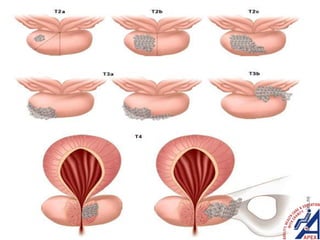
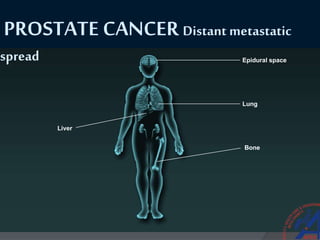
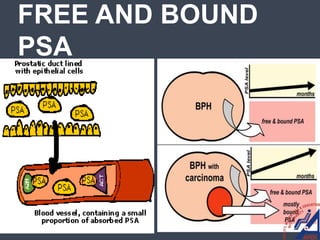
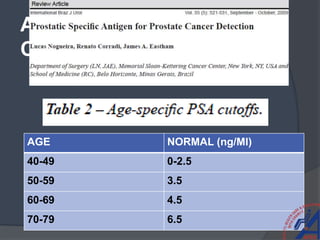
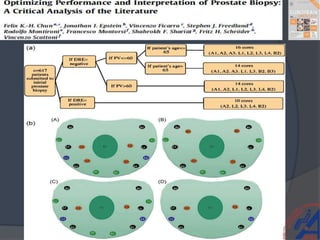
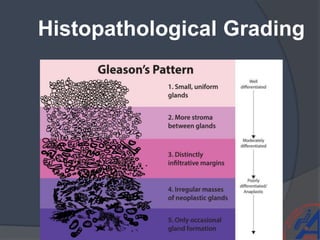
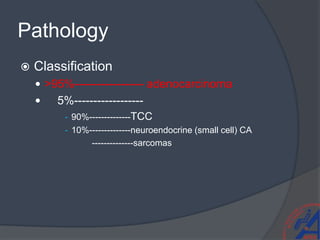
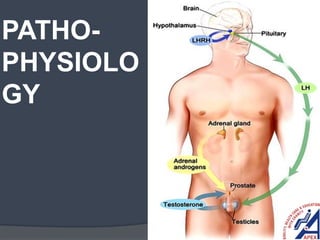
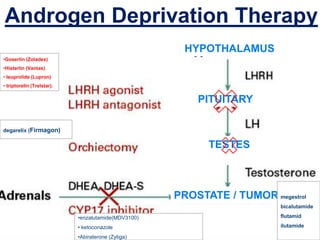
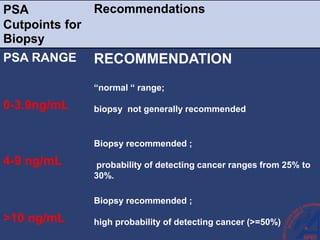
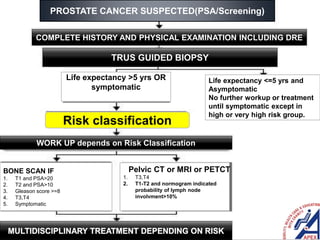
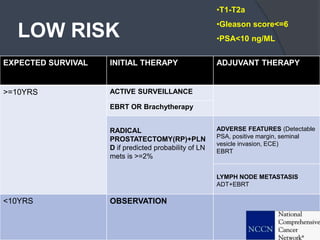
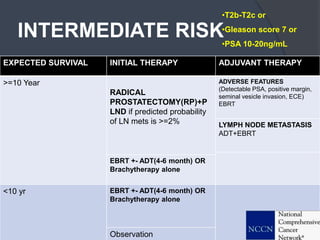
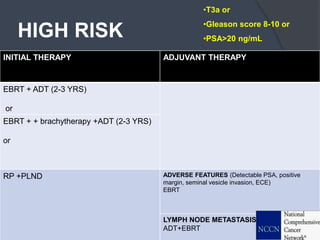
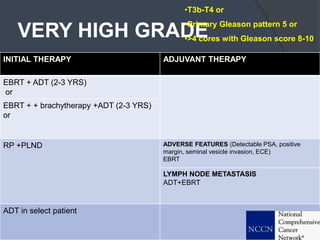
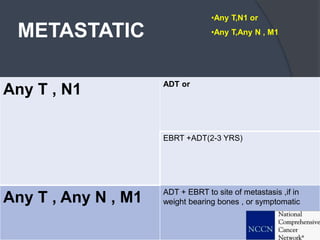
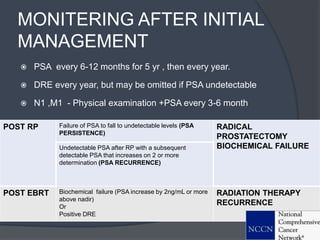
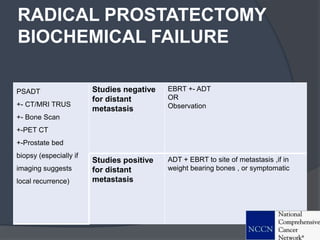
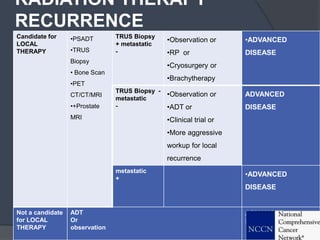
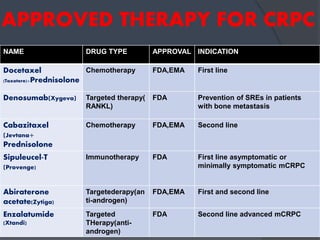
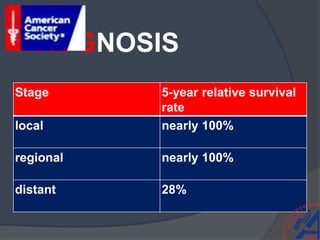
This document provides guidelines for the management of prostate cancer. It discusses the incidence, risk factors, screening guidelines, staging, grading, diagnostic testing, treatment options, and prognosis for prostate cancer. Treatment approaches are stratified based on risk classification as very low, low, intermediate, high, very high risk, metastatic, castration-resistant, and advanced disease. Management involves active surveillance, surgery, radiation therapy, hormone therapy, chemotherapy, immunotherapy, and clinical trials depending on the risk level and stage of the cancer. Prognosis depends on the stage, with 5-year survival rates of nearly 100% for local or regional disease and 28% for distant metastases.