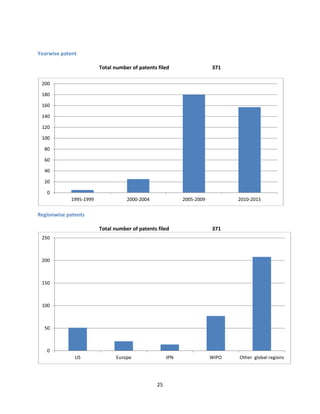
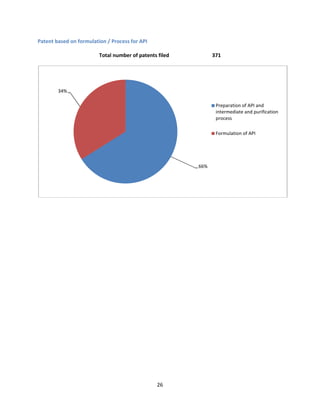
The document provides a comprehensive profile of olmesartan, an angiotensin II receptor antagonist used primarily for treating hypertension. It covers key aspects including pharmacokinetics, clinical uses, side effects, precautions, and drug interactions, as well as the demand-supply scenario in India and globally. Additionally, it details the patent situation and comparisons with other antihypertensive medications.








![19
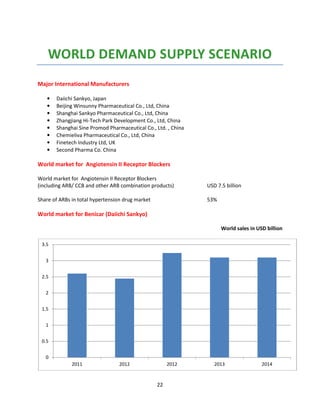
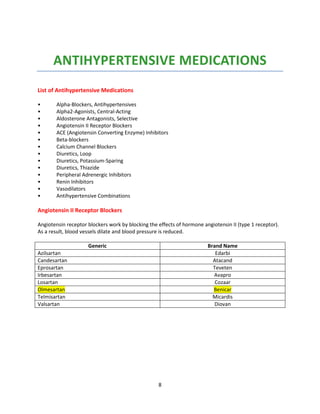
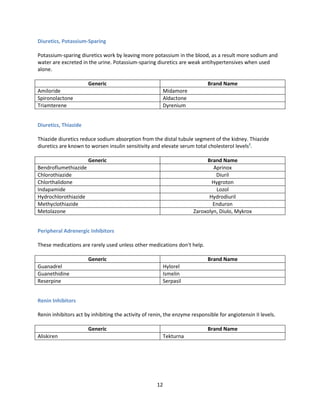
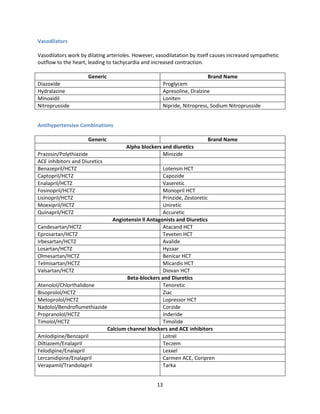
Indian formulators of Olmesartan
Name of the formulator Brand name Active Pharma Ingredients
Aamorb (Xeena) OUKAT Olmesartan
Aamorb (Xeena) OUKAT-H Olmesartan
AHPL WINBP – AM Olmesartan
AHPL WINBP- H Olmesartan
AHPL WINBP Olmesartan
AHPL WINBP TRIO Olmesartan
Ajanta ORTAN Olmesartan
AstraZeneca OLWAYS Olmesartan
AstraZeneca OLWAYS Olmesartan
Avyukt OLMISTAR Olmesartan
Avyukt OLMISTAR-H Olmesartan
Biocon OLMESAT Olmesartan
Biocon OLMESAT-AM Olmesartan
Biocon OLMESAT-ID Olmesartan
Cadila OLVAS Olmesartan
Cadila OLVAS Olmesartan
CE-Biotec OLMECARE Olmesartan
CE-Biotec OLMECARE-H Olmesartan
Cipla OLMECIP Olmesartan
Cipla OLMECIP-AM Olmesartan
Cipla OLMECIP-H Olmesartan
Cipla OLMECIP-TRIO Olmesartan
Zydus OLMY Olmesartan
Cubit Cucard) CULMI Olmesartan
Cubit (Cucard) ] CULMI-H Olmesartan
East West HYBREED-H Olmesartan
Emcure OLMEGARD Olmesartan
Emcure OLMEGARD-H Olmesartan
Eris OLMIN Olmesartan
Eris OLMIN-CH Olmesartan
Eris OLMIN-H Olmesartan
Fenestra OLMISTRA Olmesartan
Glenmark (Healtheon) OLMAX Olmesartan
Glenmark (Healtheon) OLMAX-AM Olmesartan
Glenmark (Healtheon) OLMAX-H Olmesartan
Glenmark (Healtheon) - OLMAX-M Olmesartan
GSK BENITEC Olmesartan
GSK BENITEC-H Olmesartan
Intas OLMARK Olmesartan
Intas OLMARK-A Olmesartan
Intas OLMARK-H Olmesartan
Intas ZOLTAB Olmesartan
Intra Labs OLMEFAST Olmesartan](https://image.slidesharecdn.com/profileonolmesartan-191126181510/85/Profile-on-olmesartan-19-320.jpg)
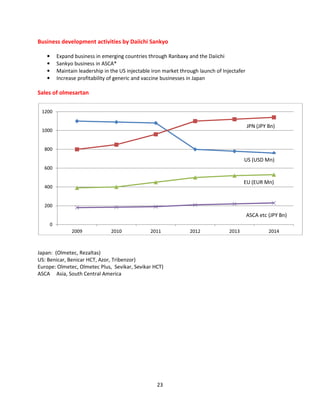
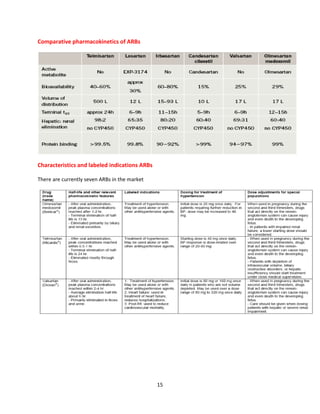
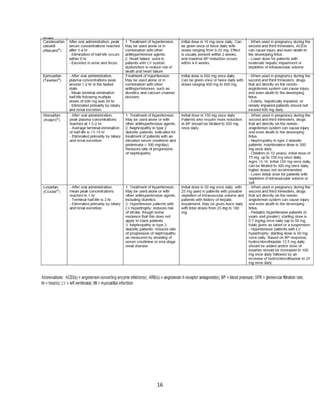
![20
Intra Labs OLMEFAST-H Olmesartan
Invision SOLMIREST Olmesartan
Invision SOLMIREST-H Olmesartan
Johnlee (Vista) OMTEN Olmesartan
Johnlee (Vista) ] OMTEN Olmesartan
Johnlee(Vista) OMTEN-AH Olmesartan
Johnlee(Vista) OMTEN-AM Olmesartan
Johnlee(Vista) OMTEN-H Olmesartan
Lifecare OLMELIFE Olmesartan
Lifecare OLMELIFE-H Olmesartan
Lupin (Pinnacle) PINOM Olmesartan
Lupin (Pinnacle) PINOM-A Olmesartan
Lupin (Pinnacle) PINOM-H Olmesartan
Lupin (Pinnacle) PINOM-M Olmesartan
Macleods AMLOVAS-OL Amlodipine
+Olmesartan
Macleods NEXOVAS-O Cilnidipine + Olmesartan
Medoxomil
Macleods OLMESAR Olmesartan
Macleods OLMESAR-A Amlodipine + Olmesartan
Macleods OLMESAR-AV Olmesartan
Macleods OLMESAR-CH Olmesartan
Macleods OLMESAR-H Olmesartan
Macleods OLMESAR-M Olmesartan
Macleods TRINEXOVAS Chlortalidone+Cilnidipine
Olmesartan
Macleods TRIOLMESAR Olmesartan
Mankind OLMETIME-AM Olmesartan
Mankind OLMETIME-AMH Olmesartan
Mankind OLMETIME-H Olmesartan
Merck OLMIGHTY Olmesartan
Merck OLMIGHTY-AM Olmesartan
Merck OLMIGHTY-H Olmesartan
Micro Carsyon OLMAT Olmesartan
Micro Carsyon OLMAT-AM Olmesartan
Micro Carsyon OLMAT-AMH Olmesartan
Micro Carsyon OLMAT-H Olmesartan
Olcare(Cardium) O-RELATE Olmesartan
Olcare(Cardium) O-RELATE-H Olmesartan
Pax Healthcare XOLMI H Olmesartan
Race Pharma ROLMEX-AM Olmesartan
Ranbaxy OLVANCE Olmesartan
Ranbaxy OLVANCE-H Olmesartan
Ranbaxy TRIOLVANCE Olmesartan
Ranbaxy (Cardiovasculars) OL-VAMLO - Olmesartan
Shrrishti HC OLEMAR Olmesartan](https://image.slidesharecdn.com/profileonolmesartan-191126181510/85/Profile-on-olmesartan-20-320.jpg)