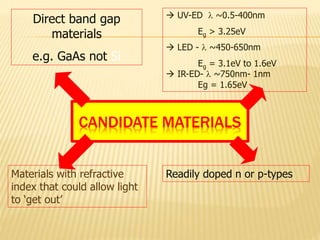
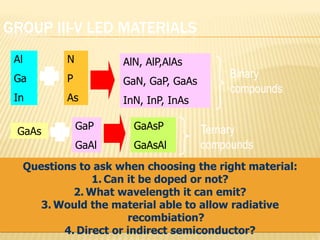
This document discusses the history and technology of LED lighting. It begins with the early sources of light from the sun and fire, through the invention of the light bulb. In the late 20th century, Shuji Nakamura invented the first blue and white LEDs, starting an LED revolution. The document then explains what an LED is - a semiconductor p-n junction that emits light when forward biased. Different semiconductor materials are used to produce light across the UV/visible/IR spectrum. Key materials for red, yellow, green and blue LEDs are gallium arsenide, gallium phosphide, and gallium nitride. The green LED was an important innovation for which Shuji Nakamura received