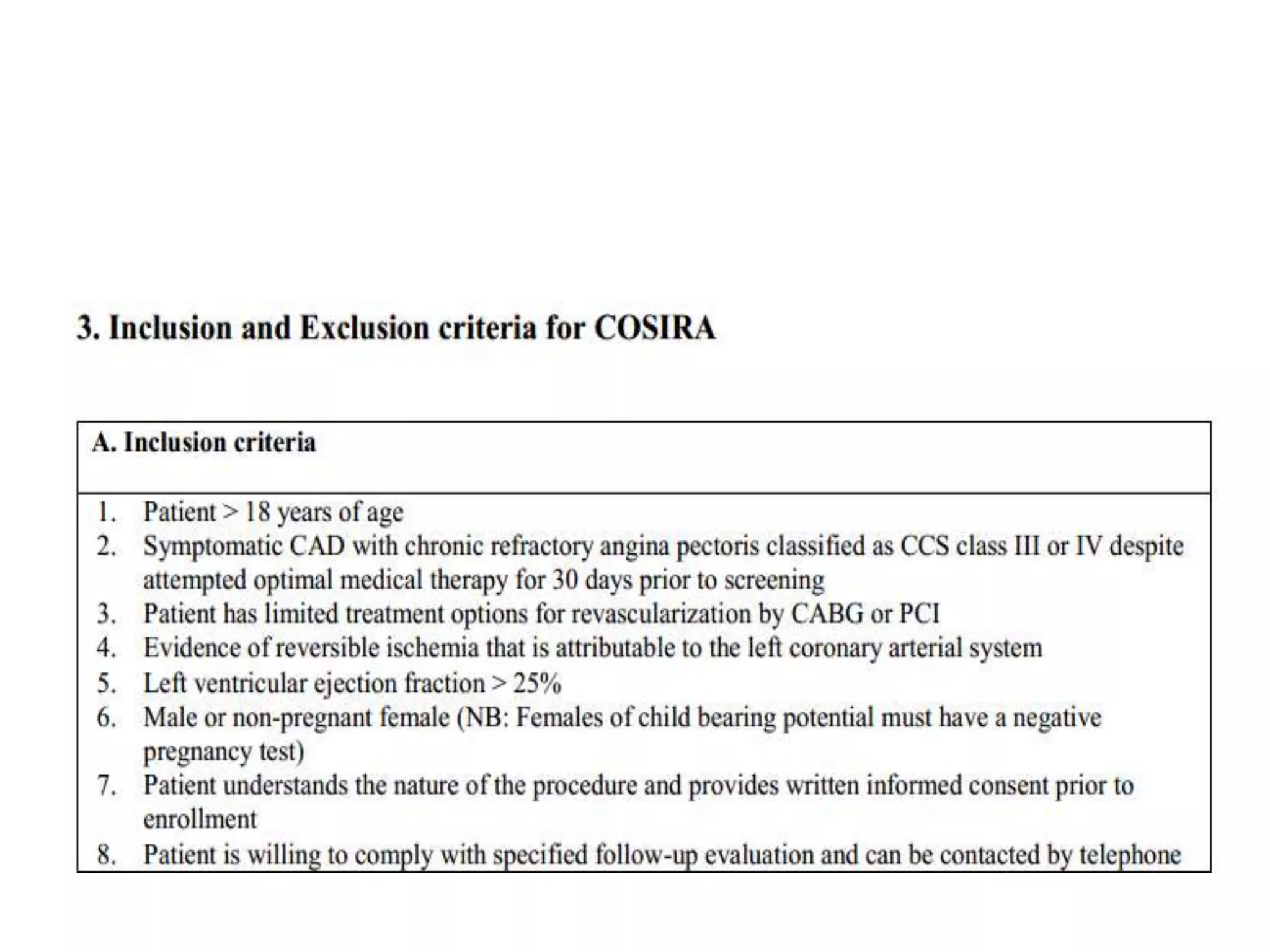
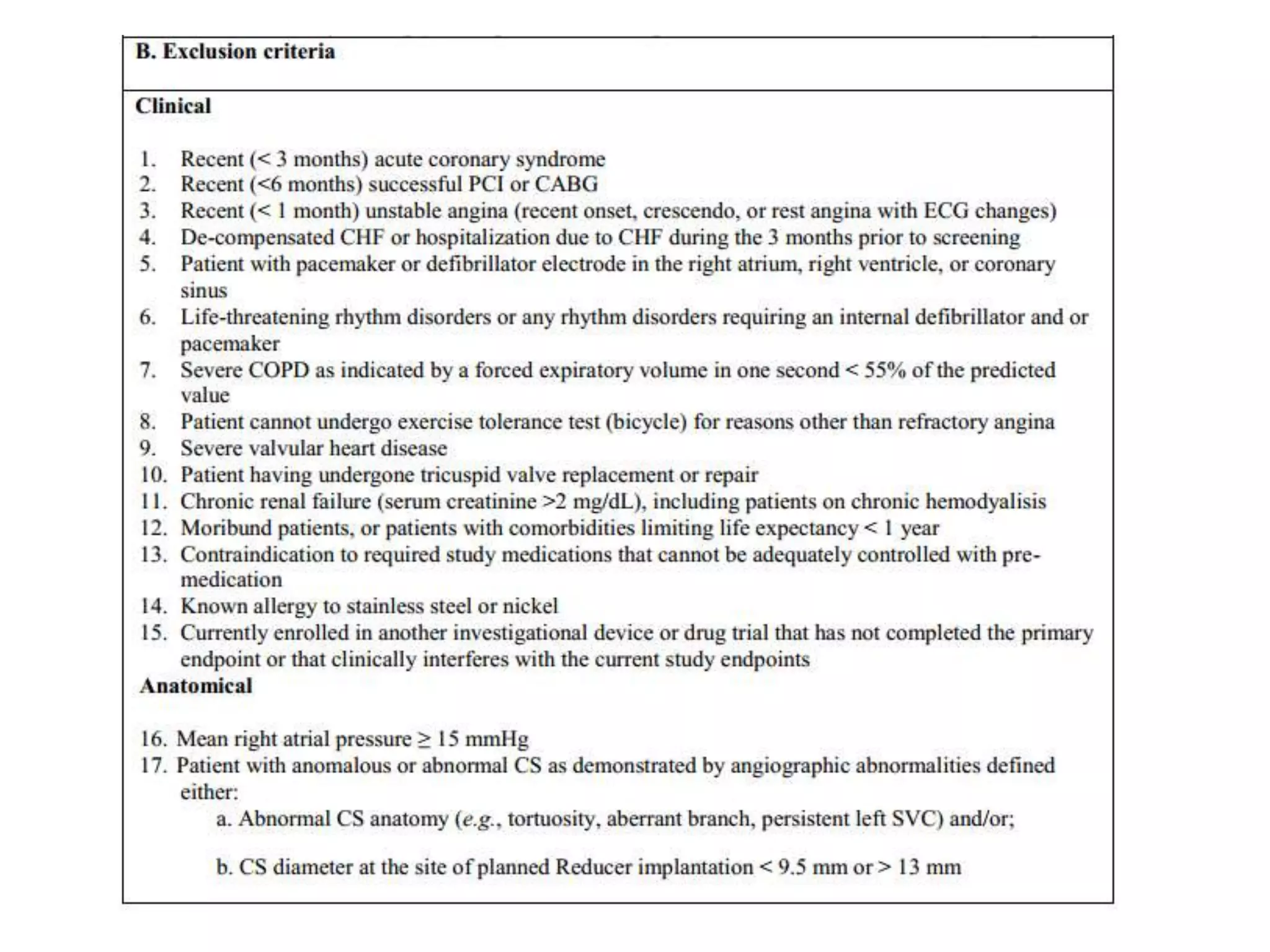
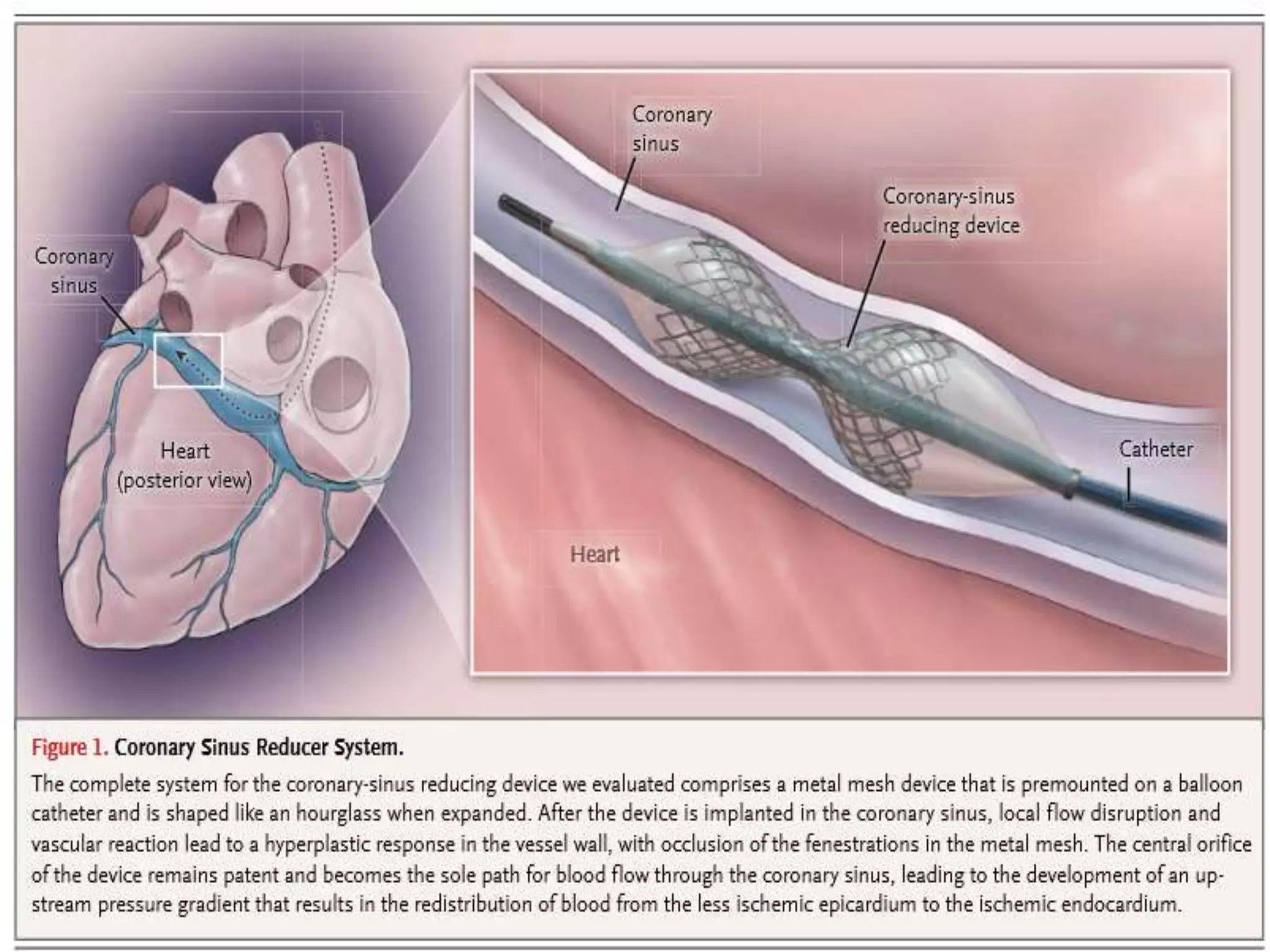
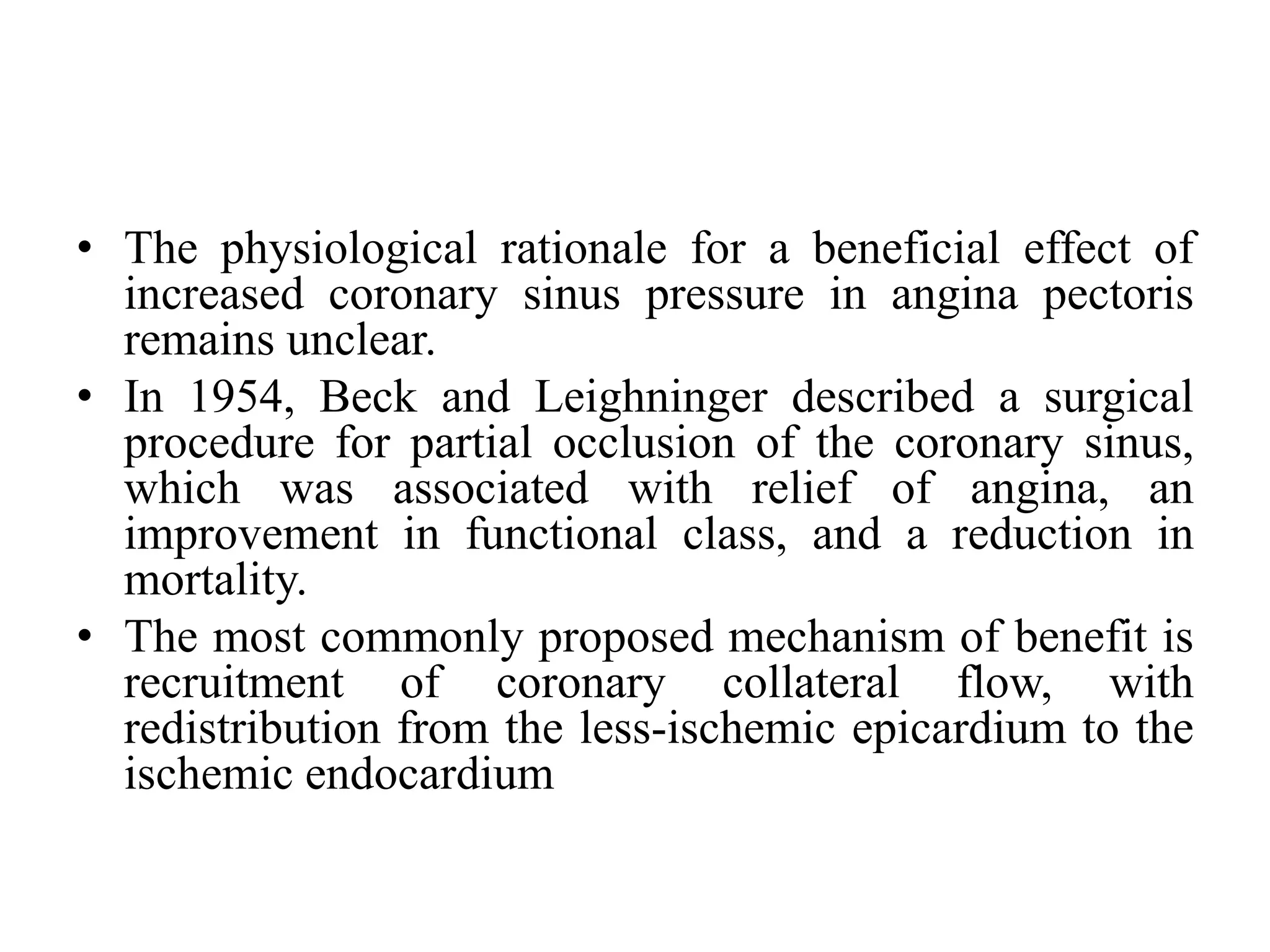
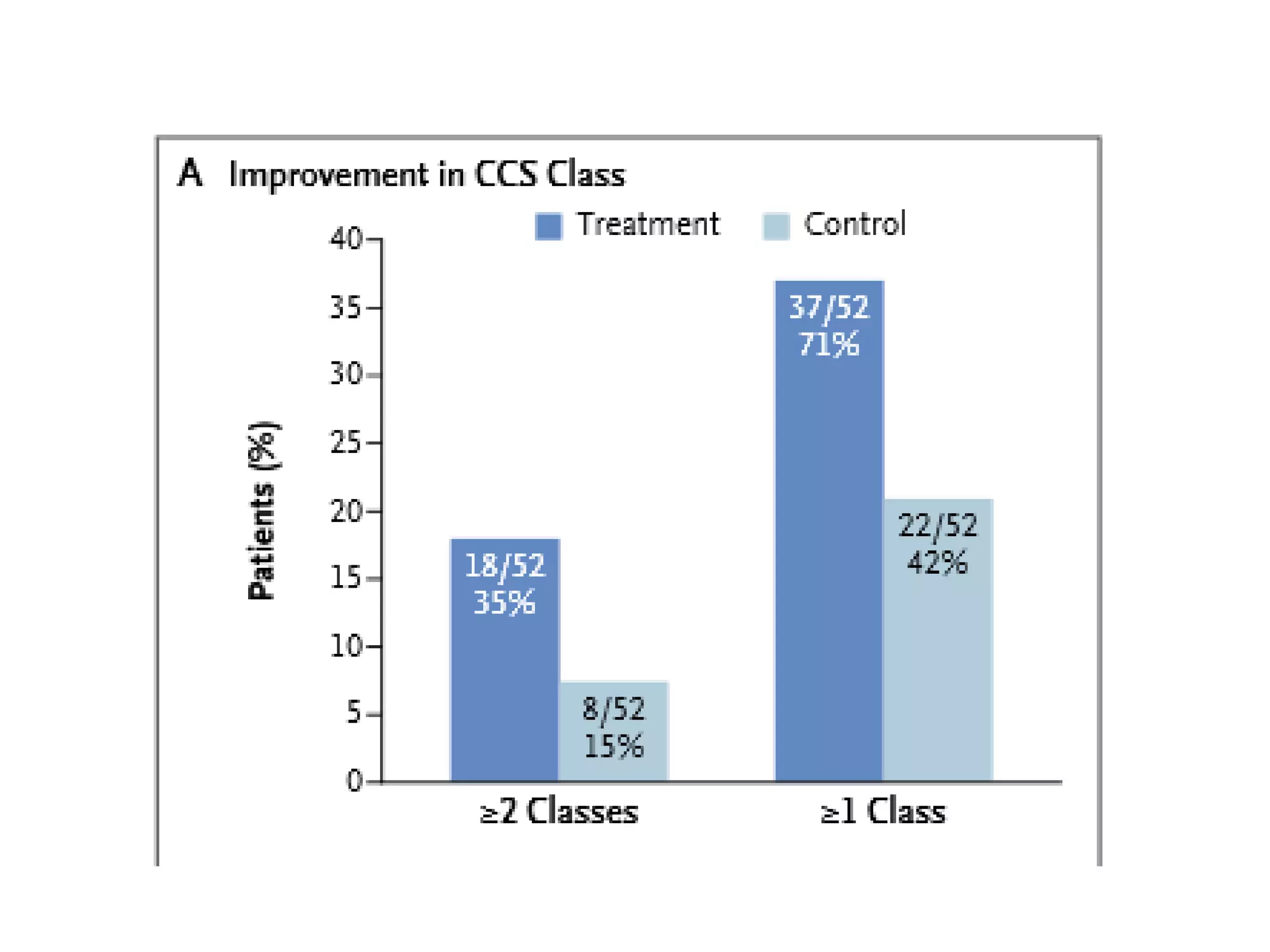
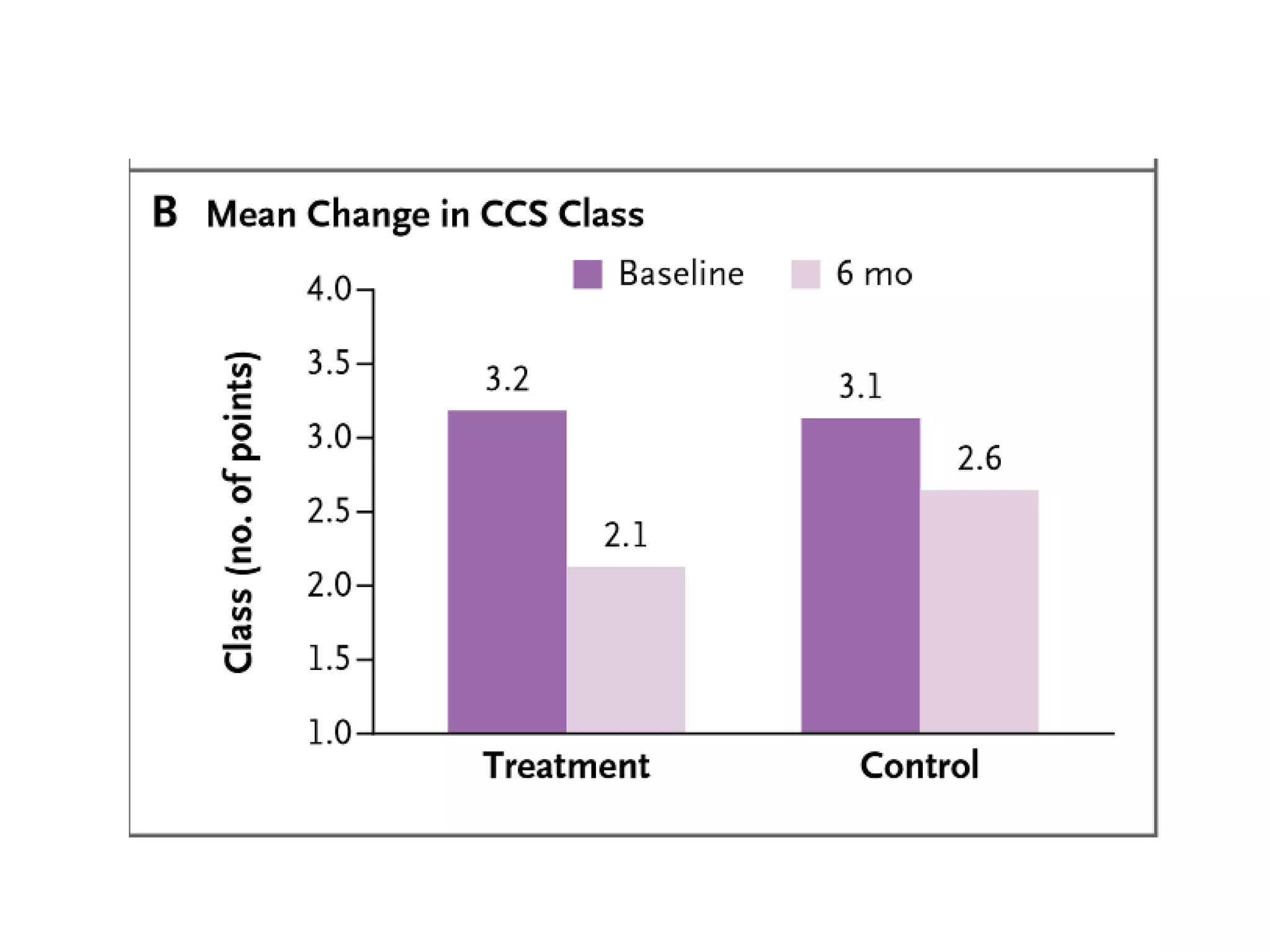
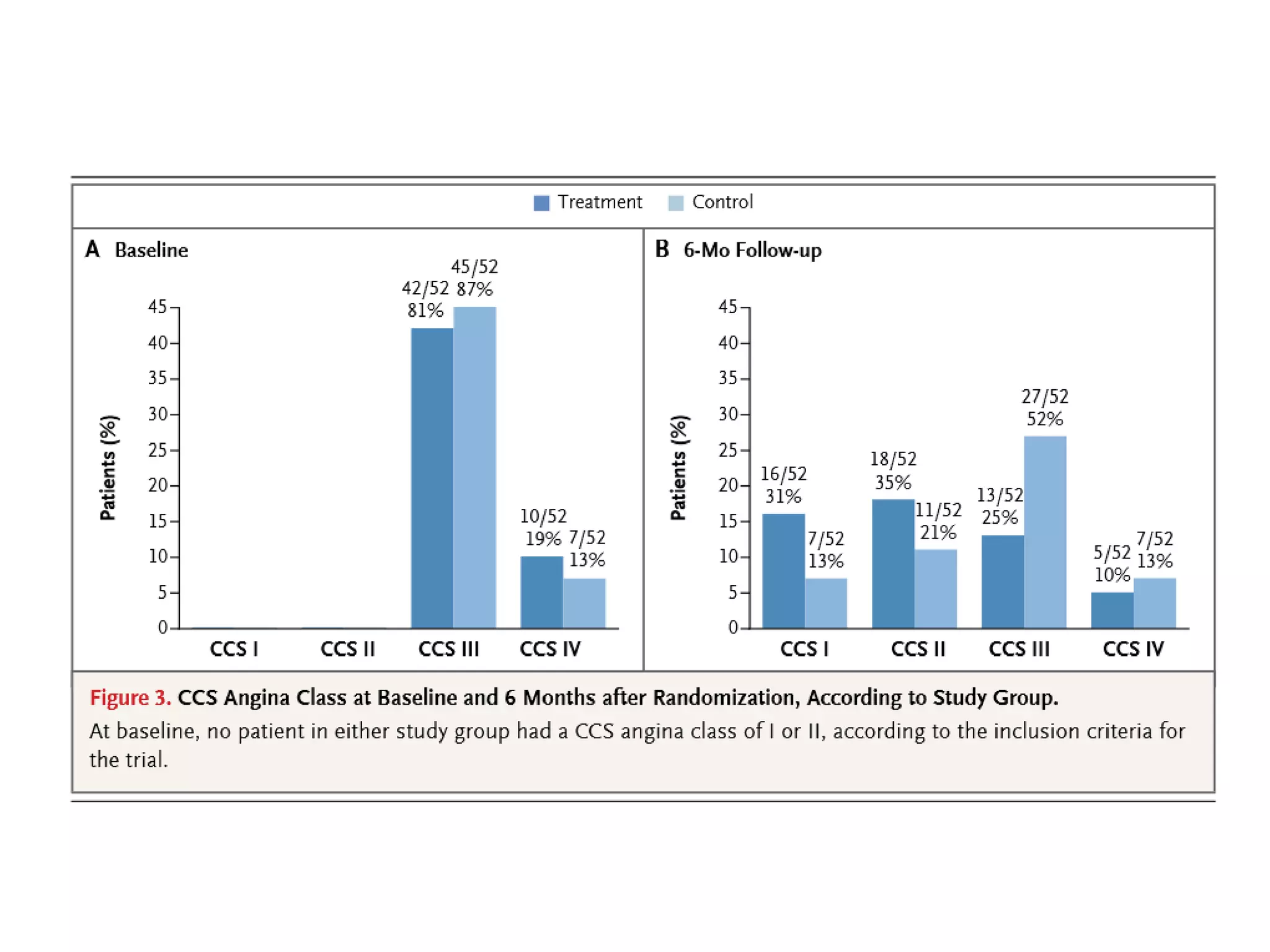
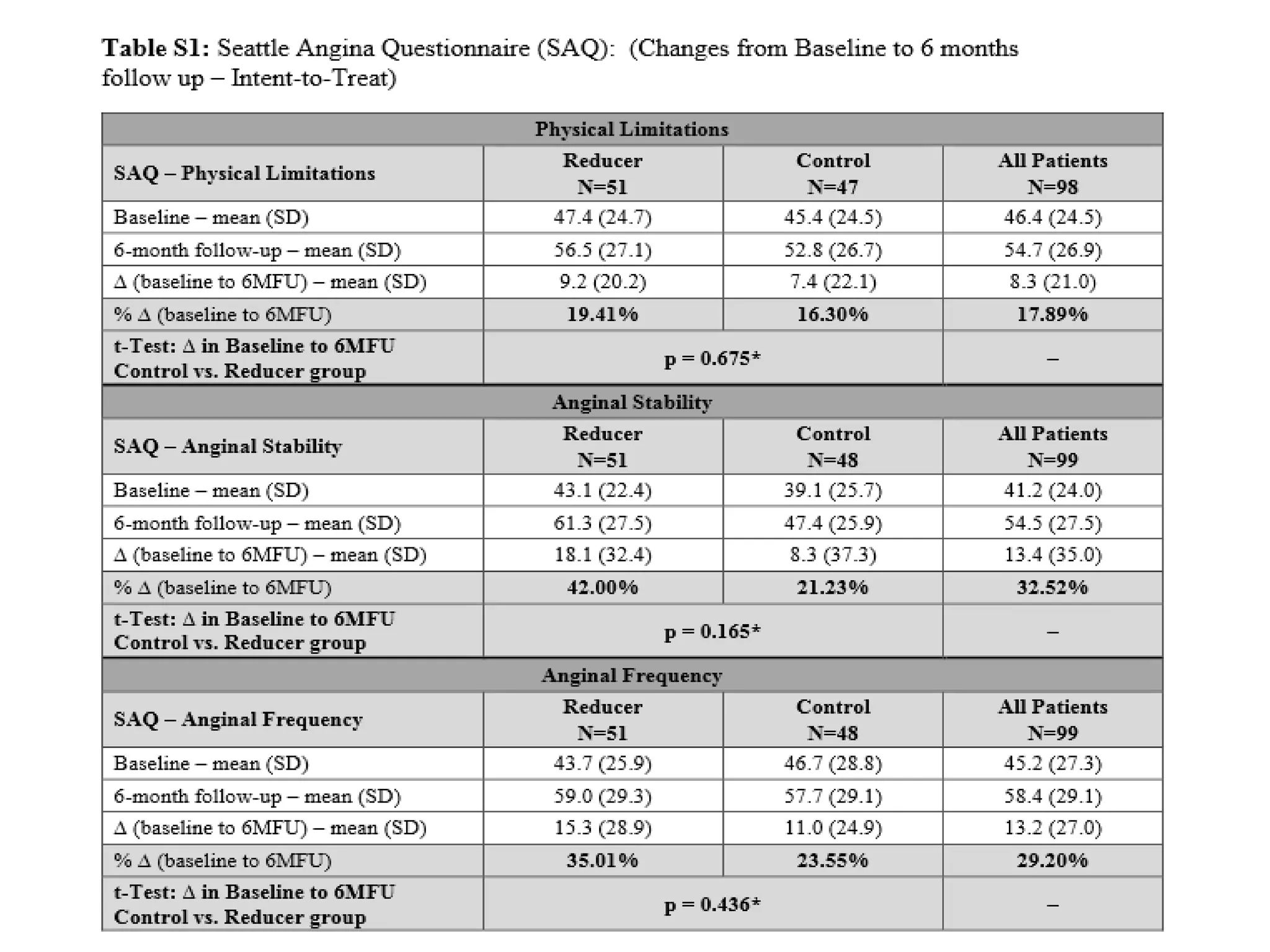
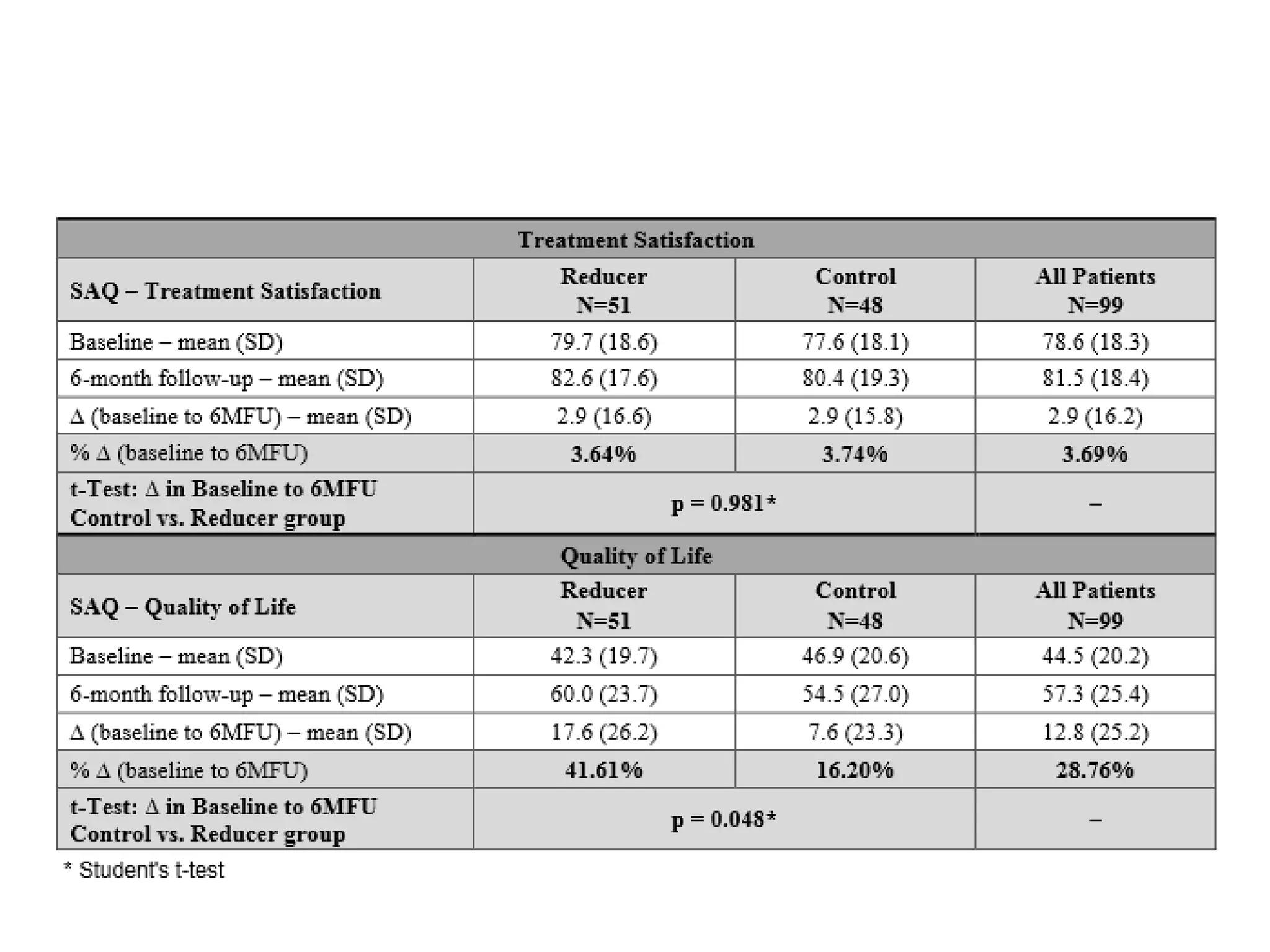
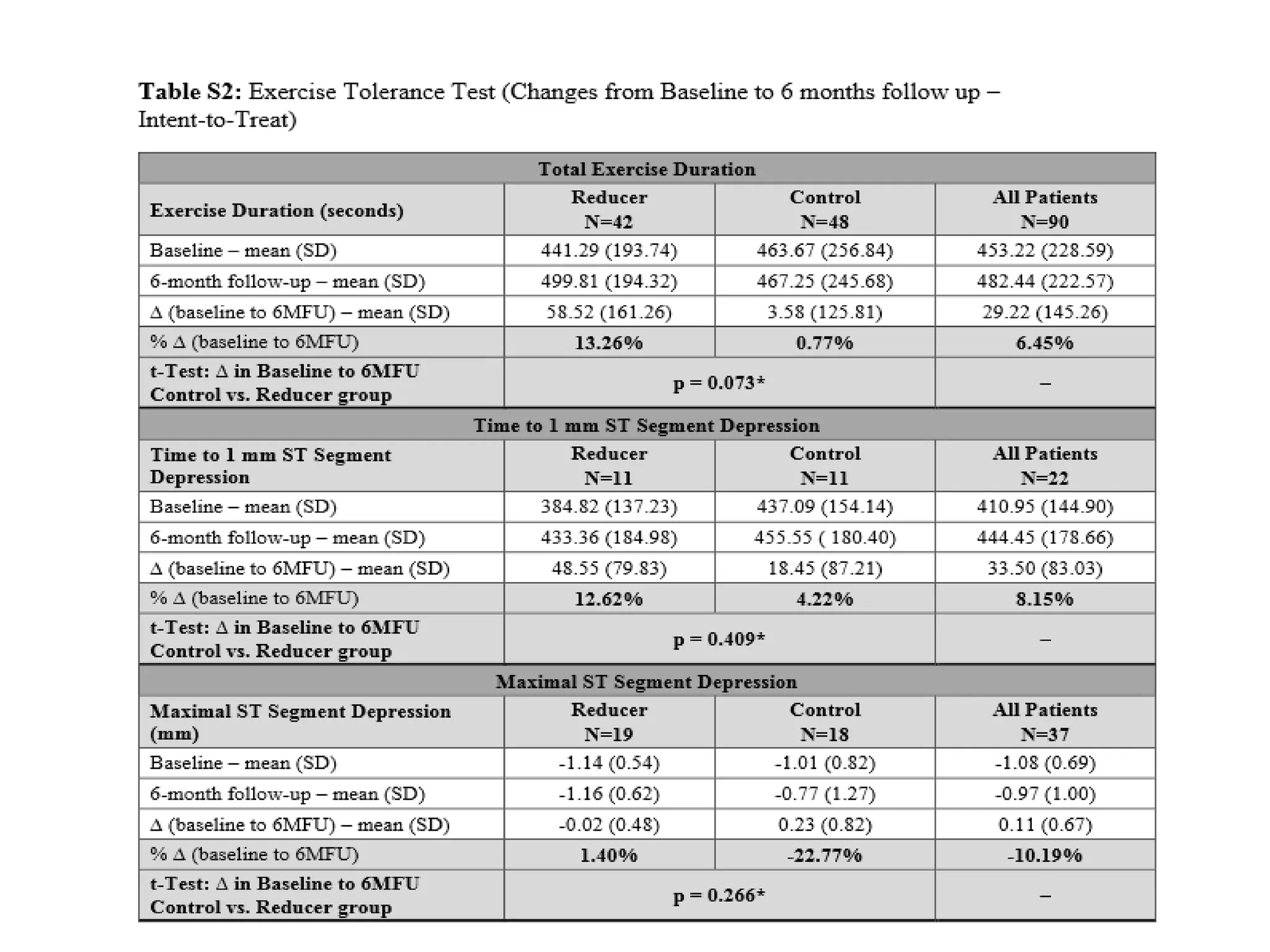
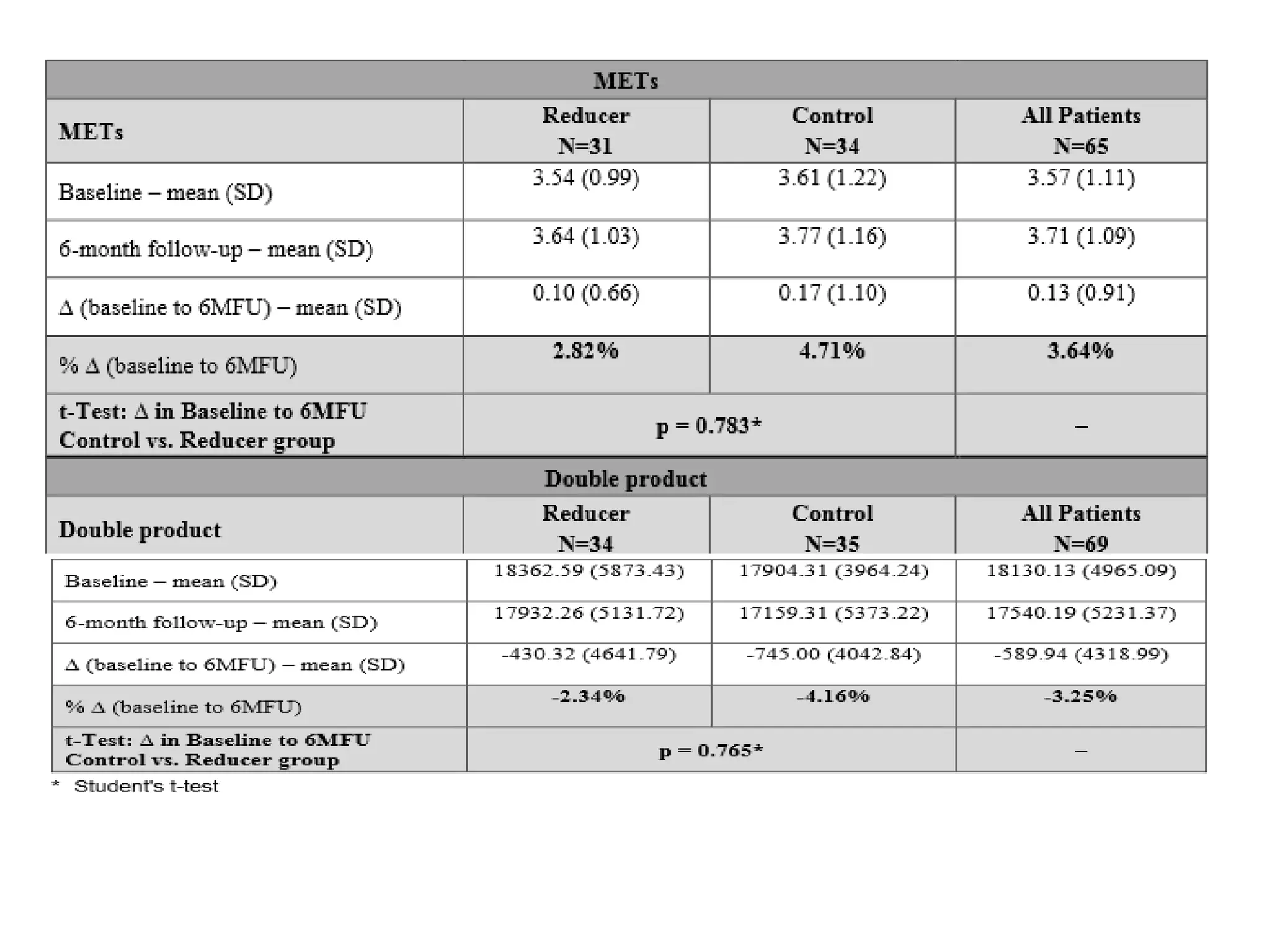
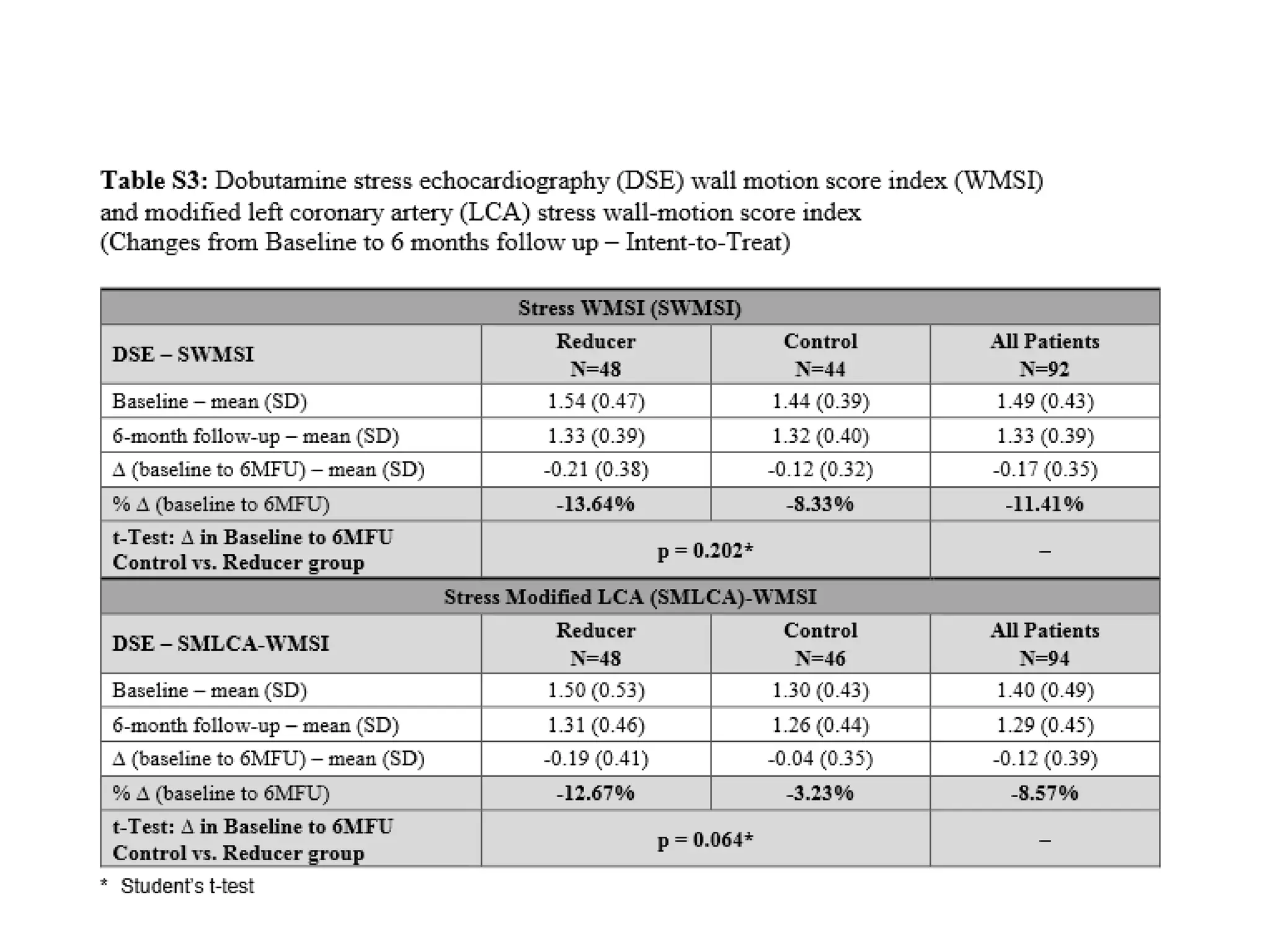
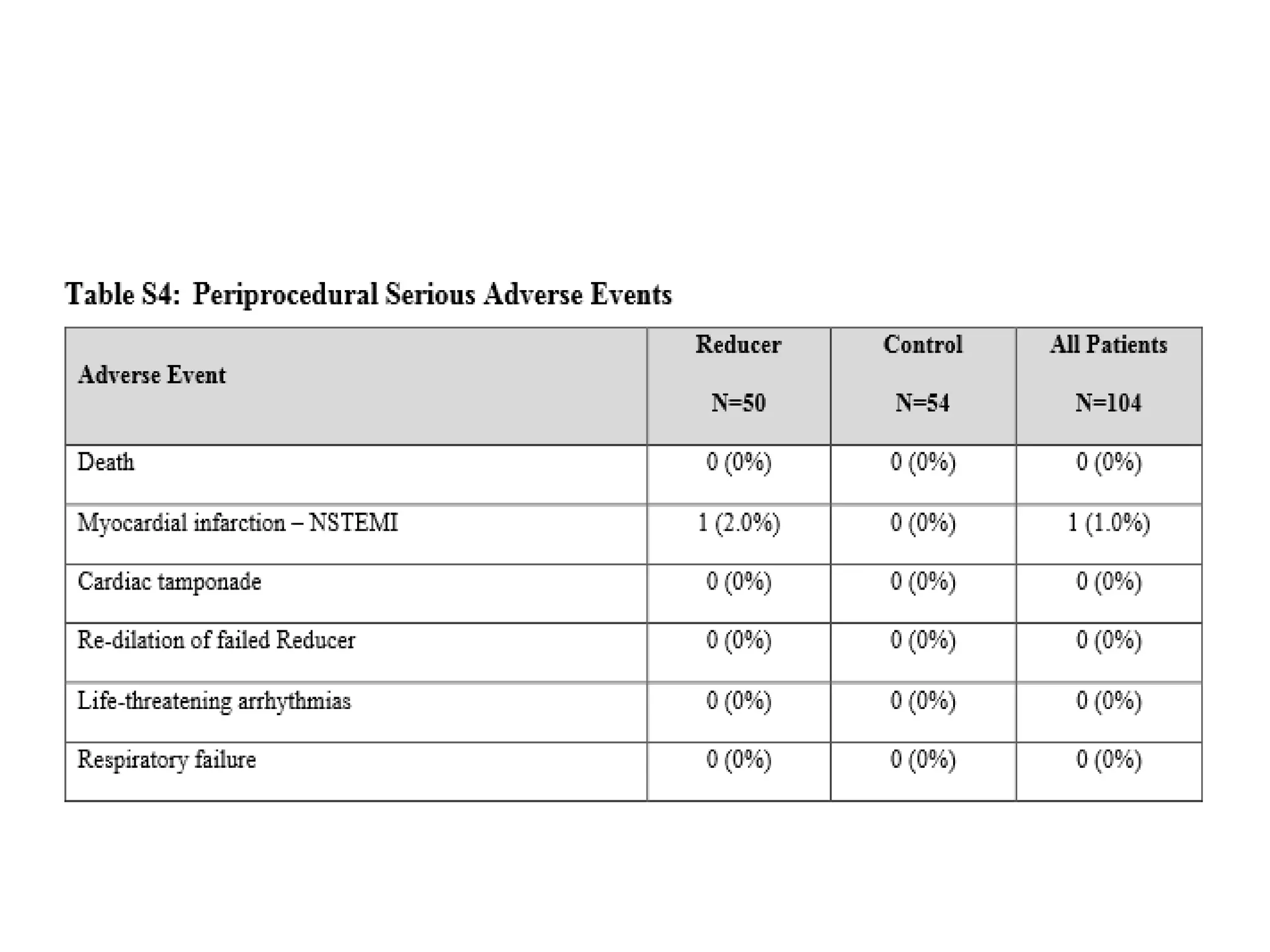
This document summarizes a clinical trial testing a coronary sinus reducing device for the treatment of refractory angina. The trial involved 104 patients randomized to receive the hourglass-shaped device, which creates pressure in the coronary sinus to redistribute blood flow, or a sham procedure. At 6-month follow-up, the device led to reduced angina symptoms and improved quality of life compared to the sham group, as measured by the Seattle Angina Questionnaire and Canadian Cardiovascular Society class. The results provide preliminary evidence that increasing coronary sinus pressure may help relieve angina by recruiting collateral blood flow.