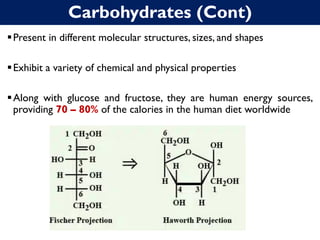
The document is a lecture on carbohydrates from a food chemistry course, outlining learning outcomes related to their properties, classifications, and roles in nutrition. It explains different types of carbohydrates, such as monosaccharides and disaccharides, their chemical structures, and functions in the human body. Key concepts include the importance of carbohydrates as energy sources and their synthesis in plants through photosynthesis.