1. A patient was prescribed the wrong dose of phenytoin due to brand substitution and received 1025 mg instead of 270 mg, resulting in abnormal CT changes and prolonged drowsiness.
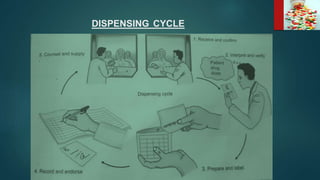
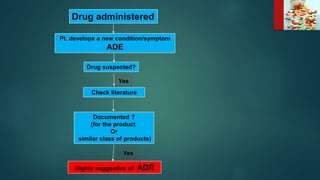
2. Dispensing and administration errors can occur from issues like confusing drug names, incorrect dosages, wrong drugs or routes of administration.
3. It is important for healthcare providers to have proper training, follow safe procedures, double check medications, and report any errors to prevent harm to patients.