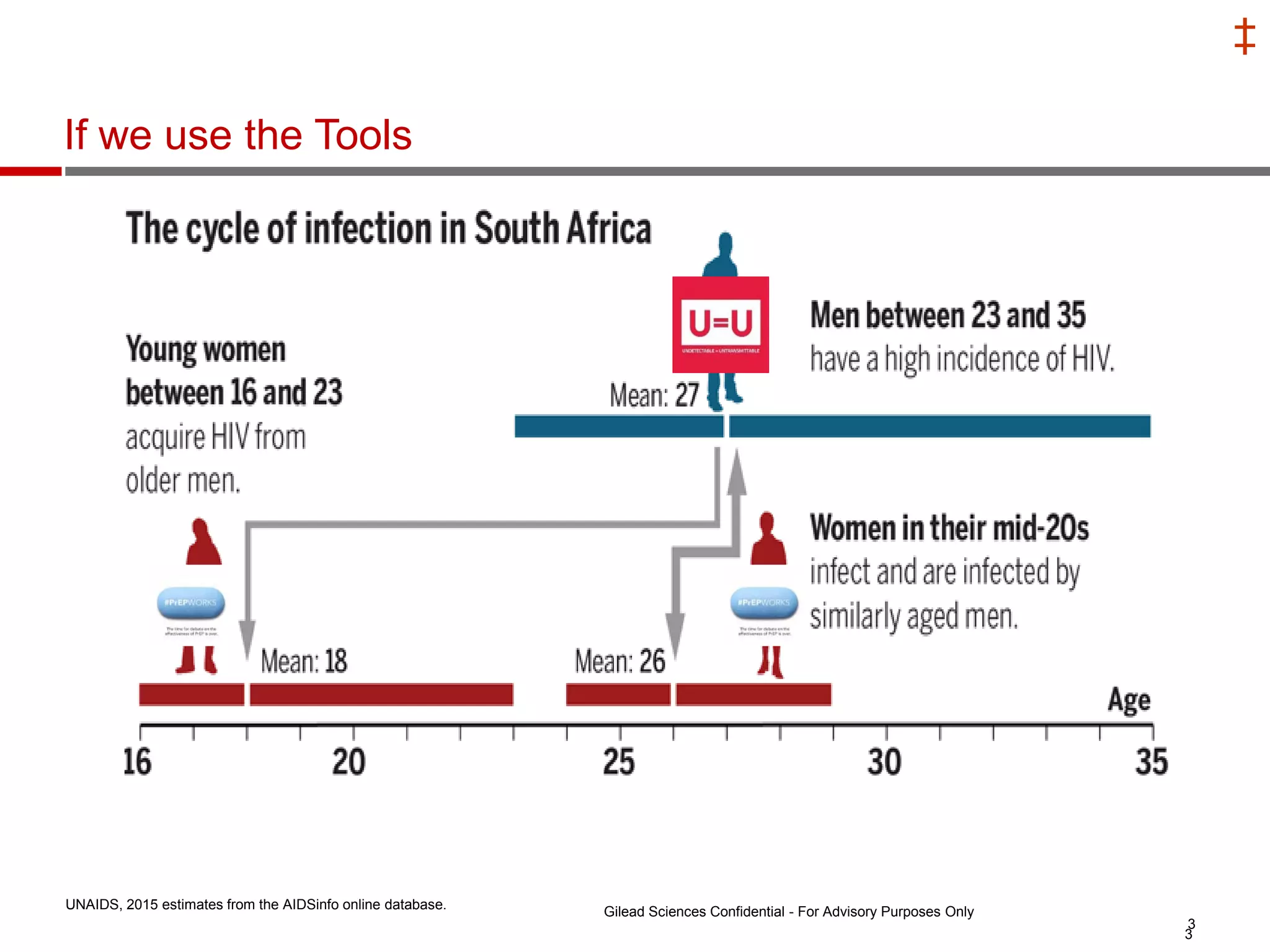
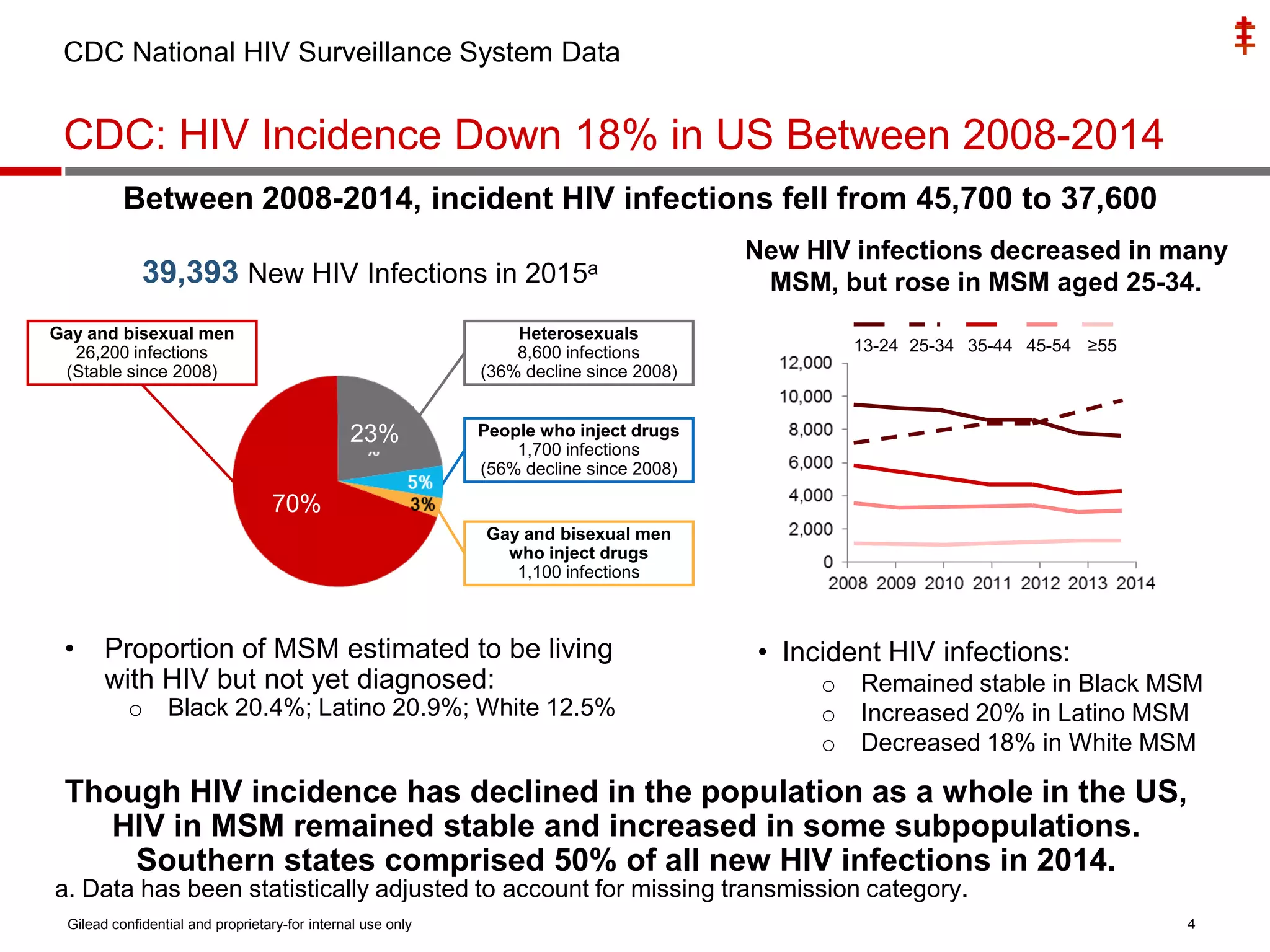
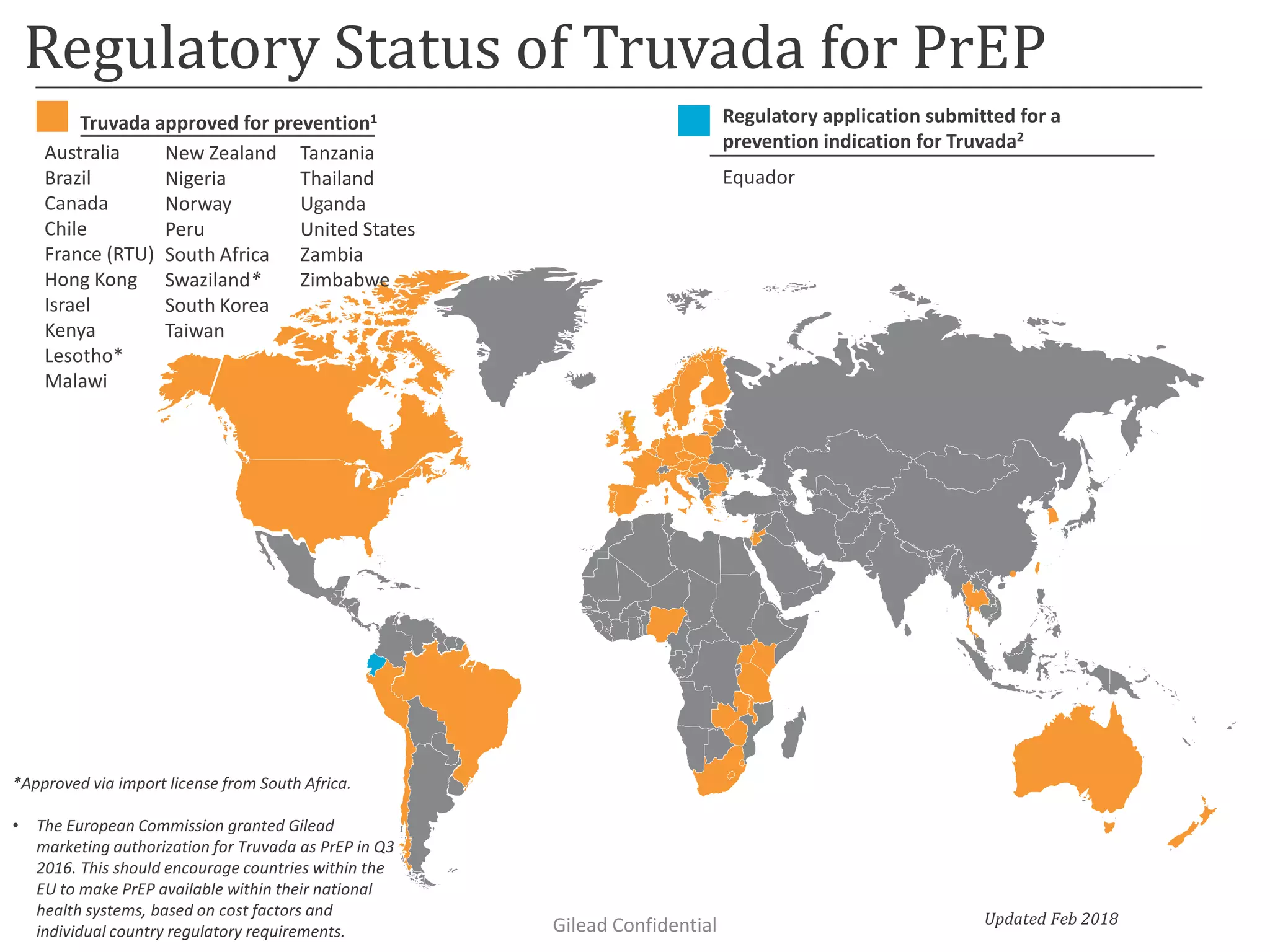
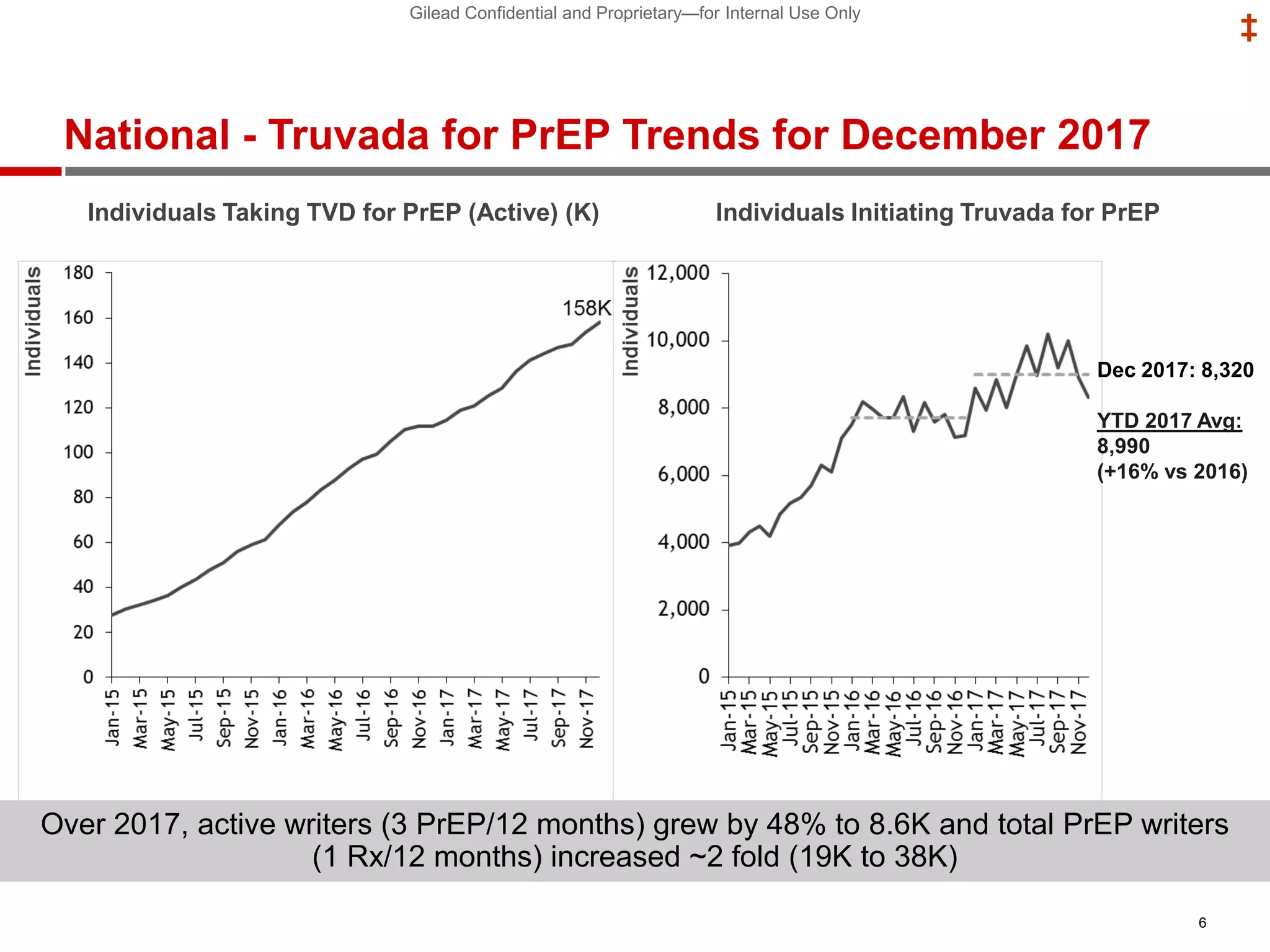
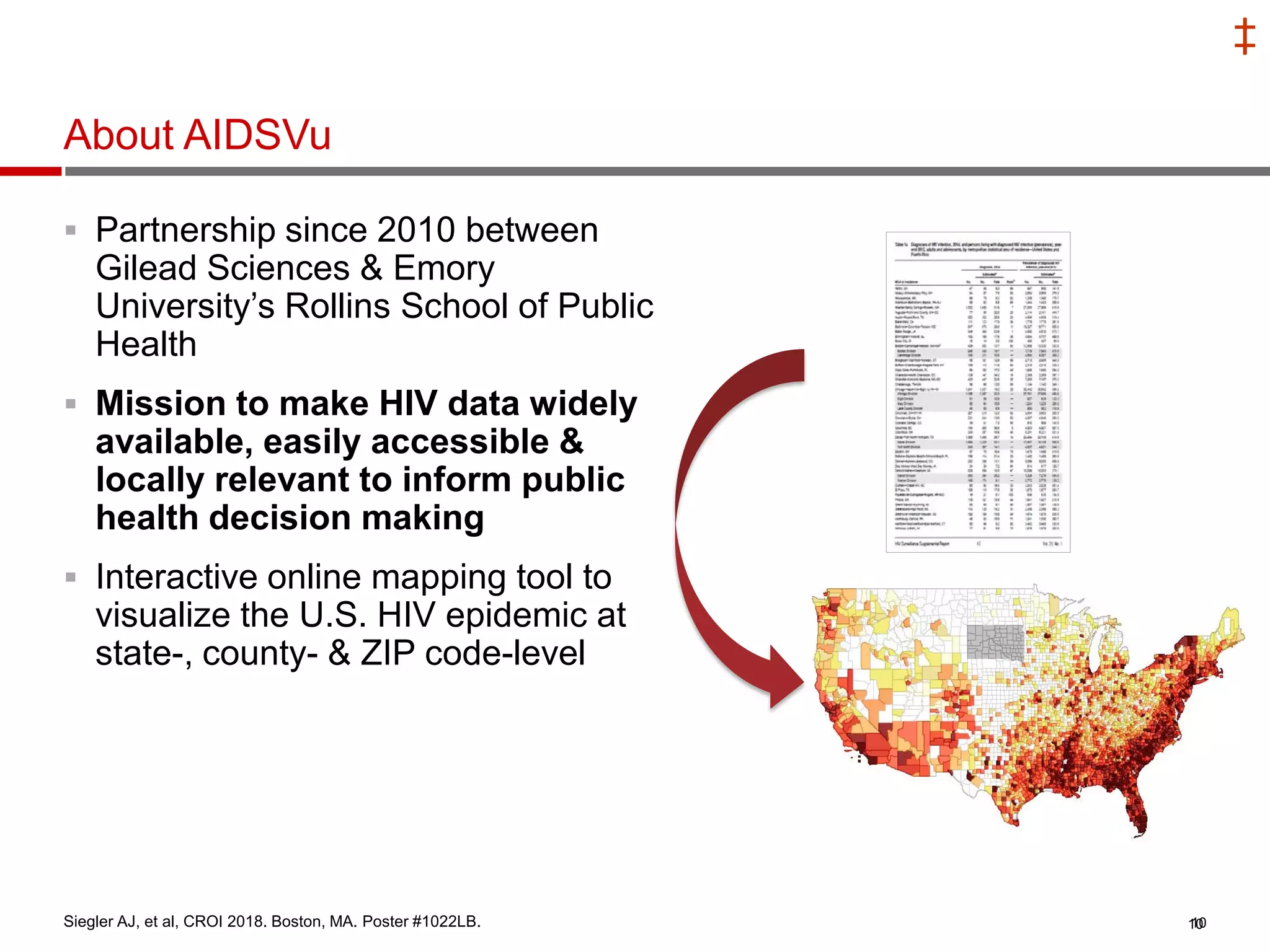
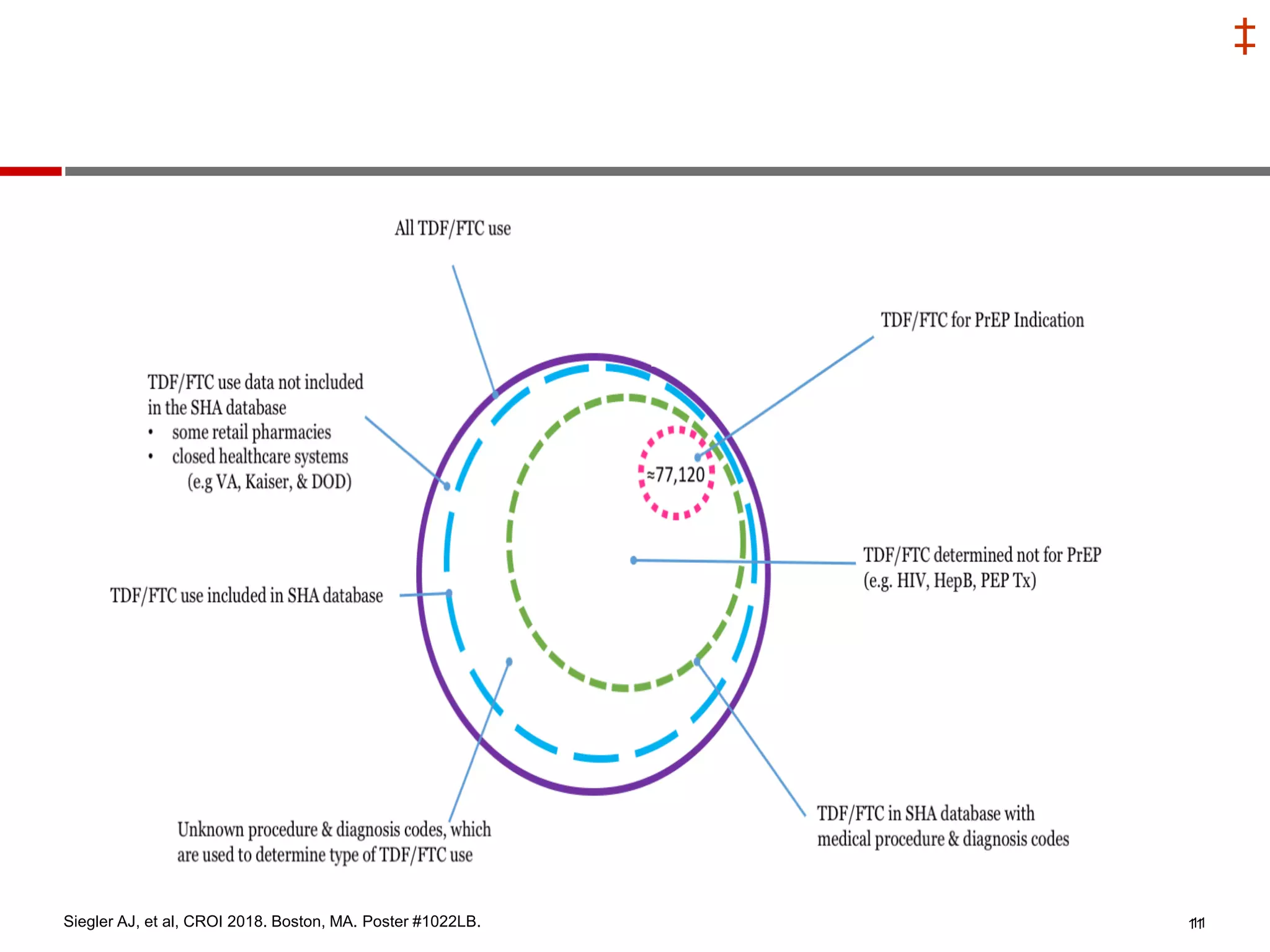
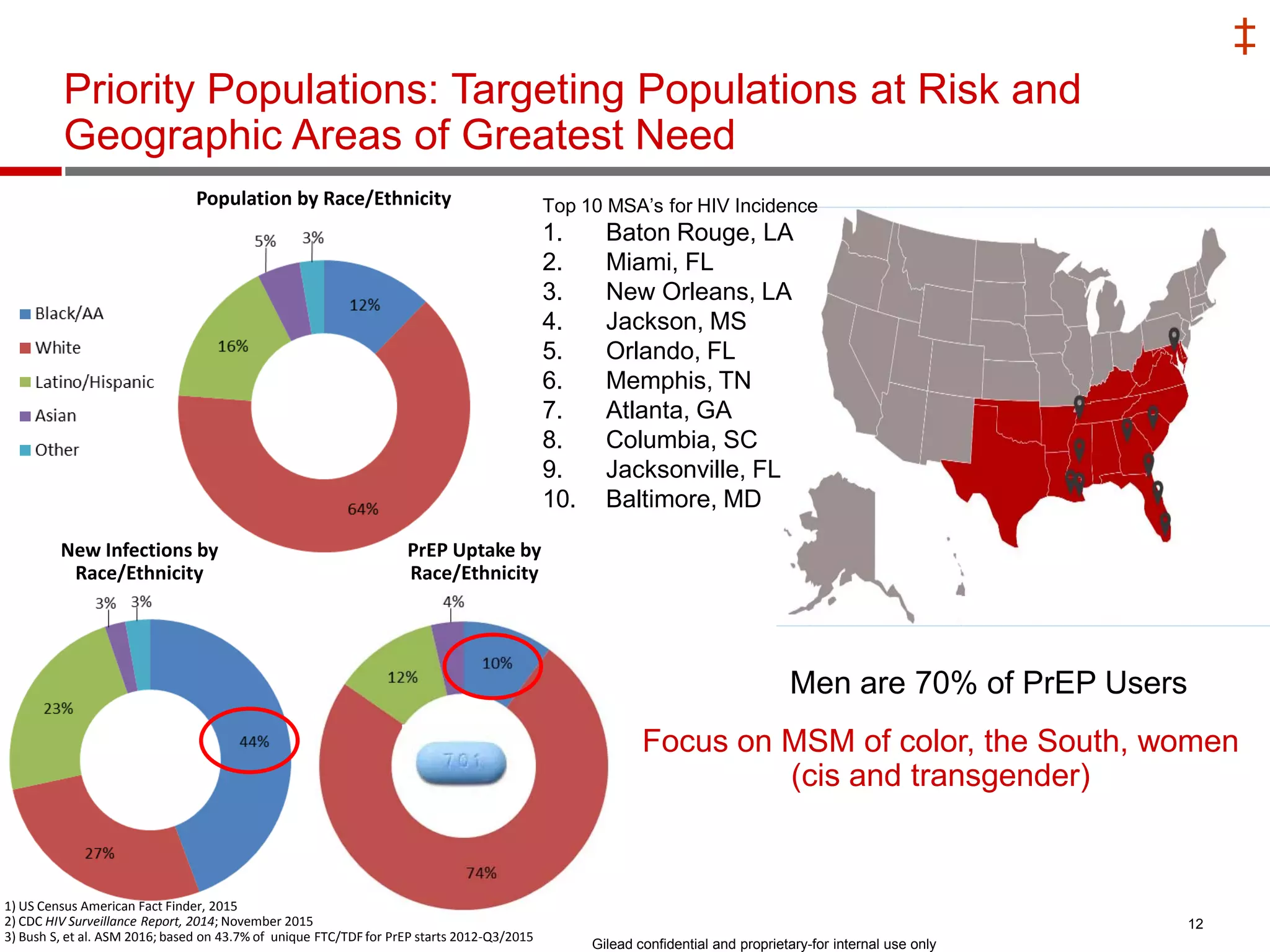
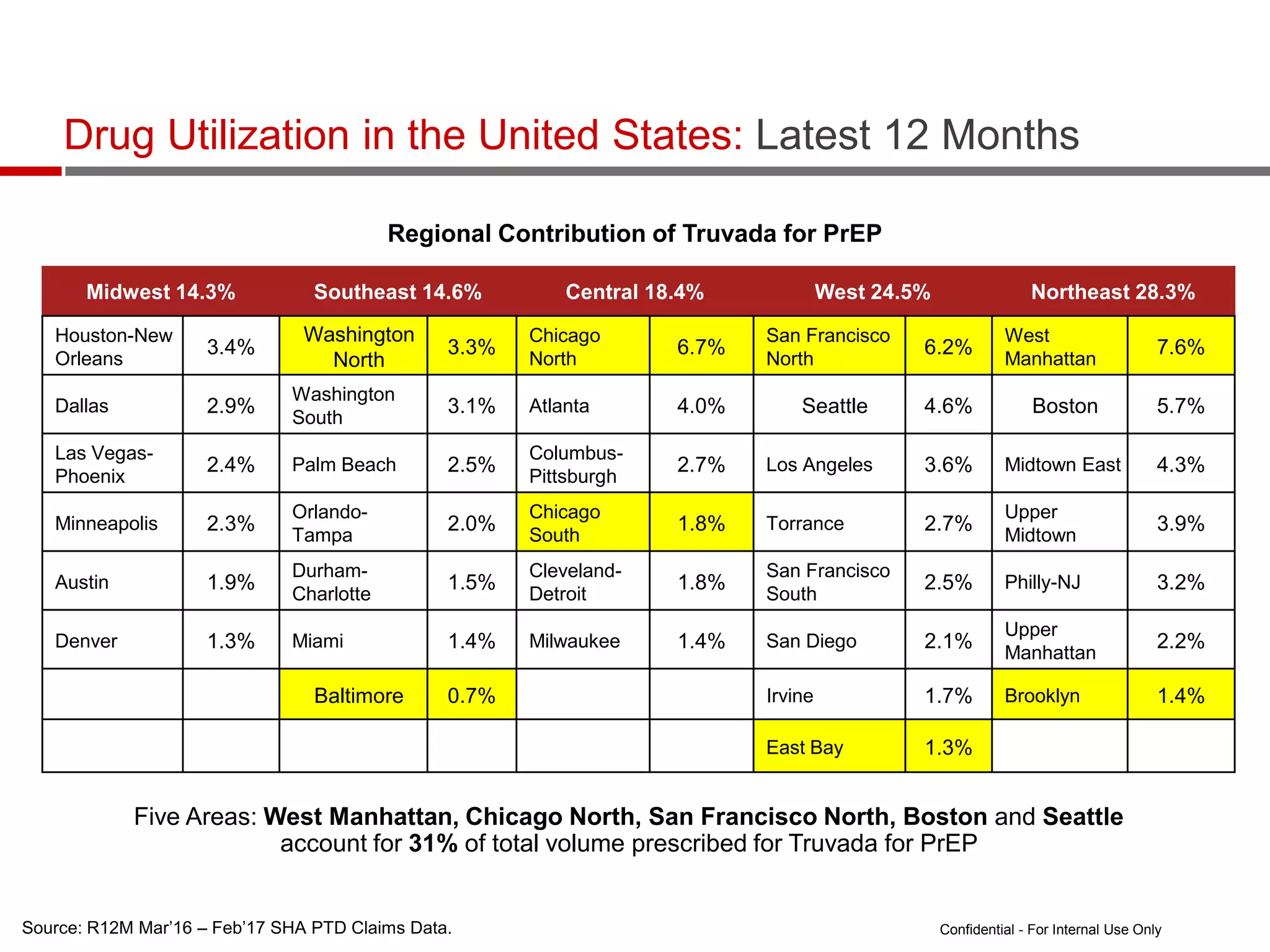
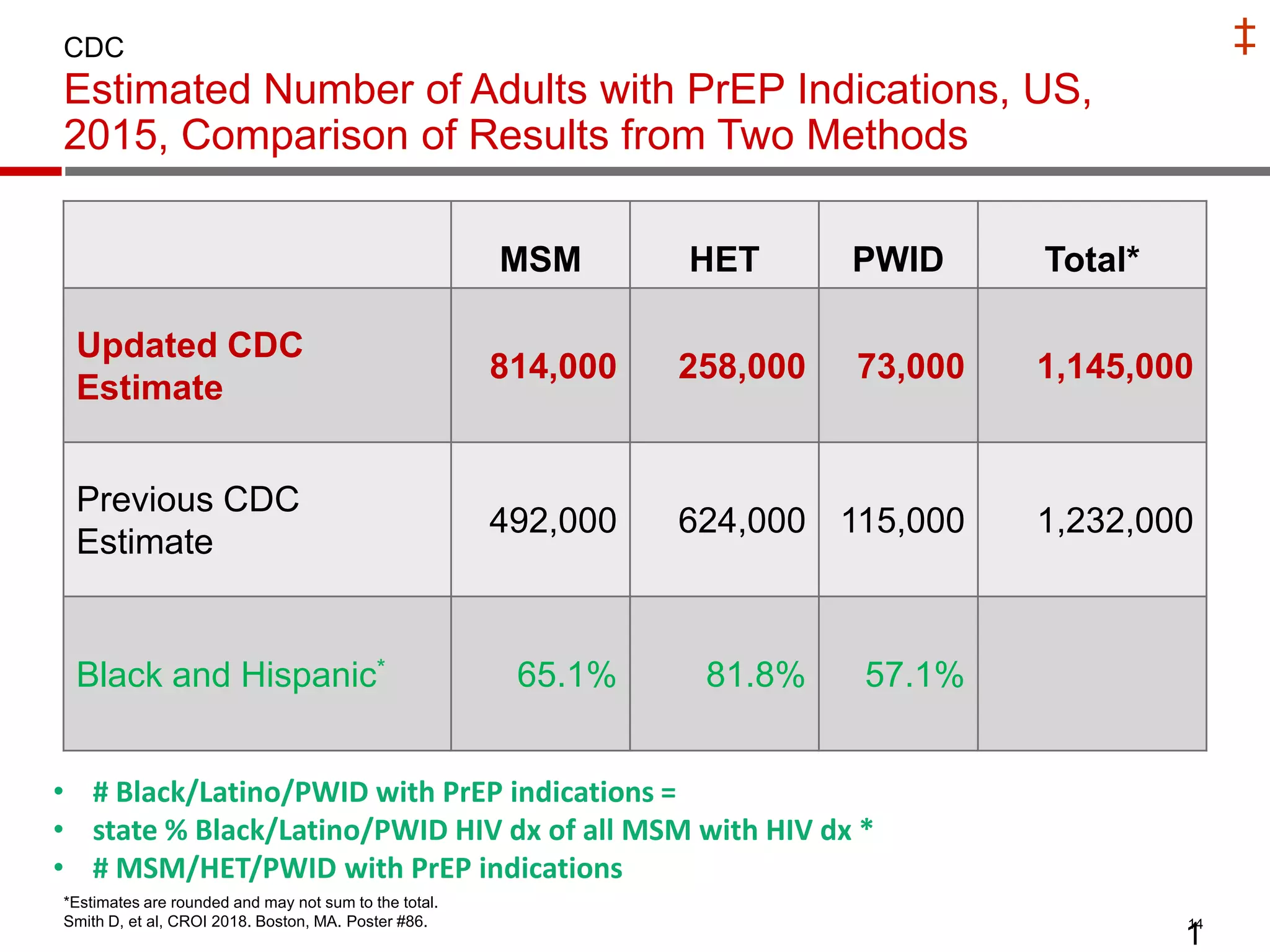
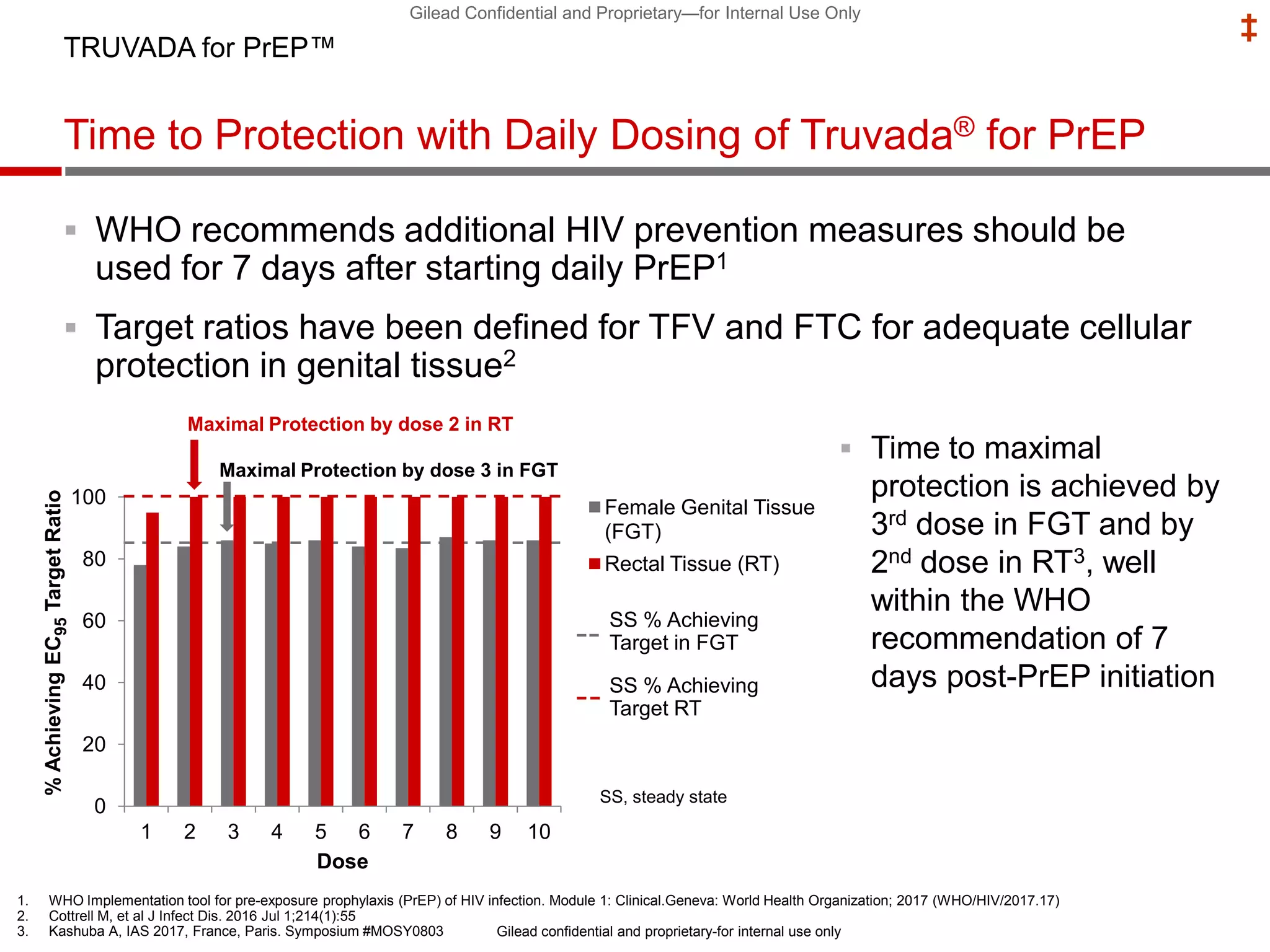
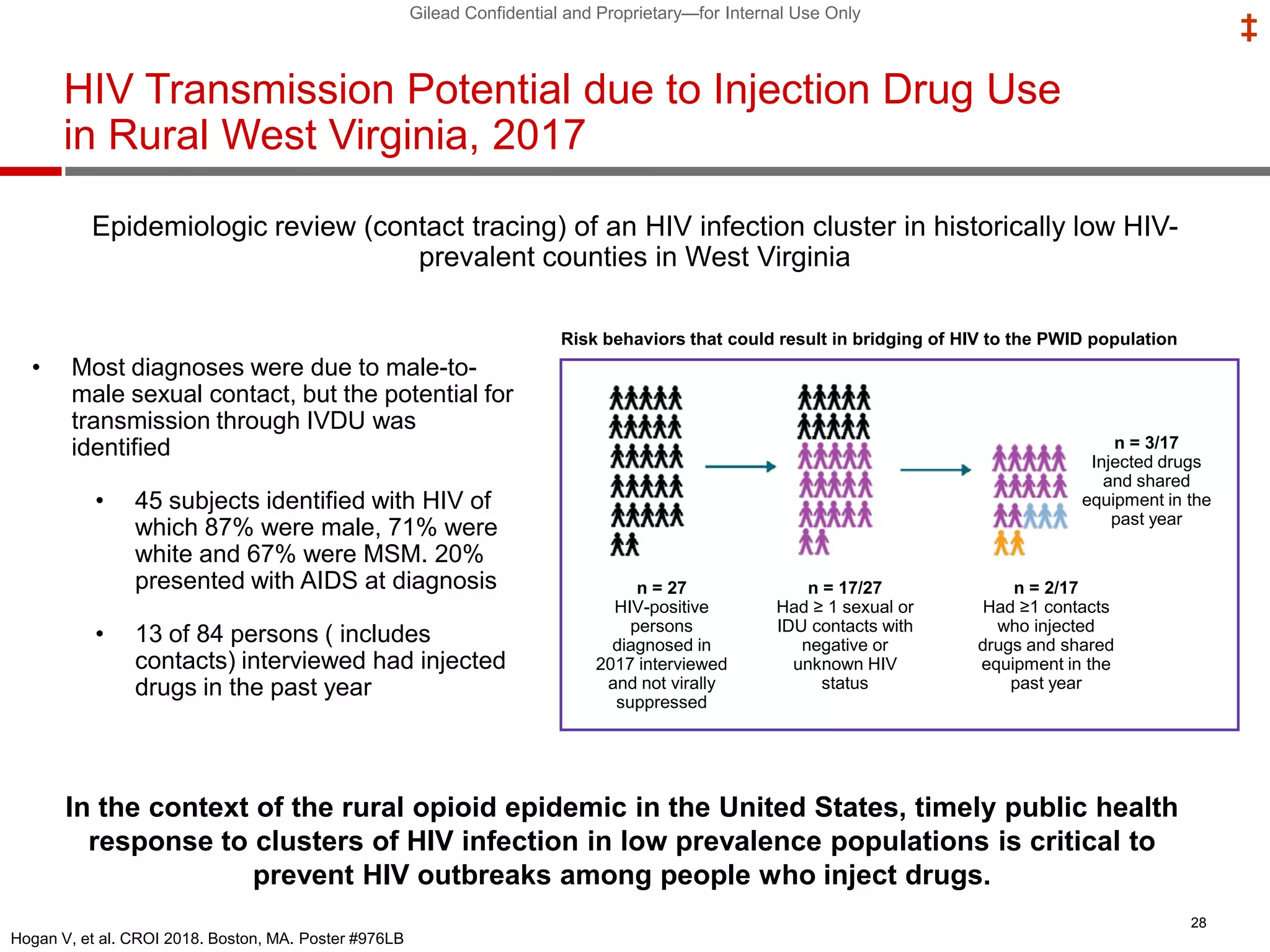
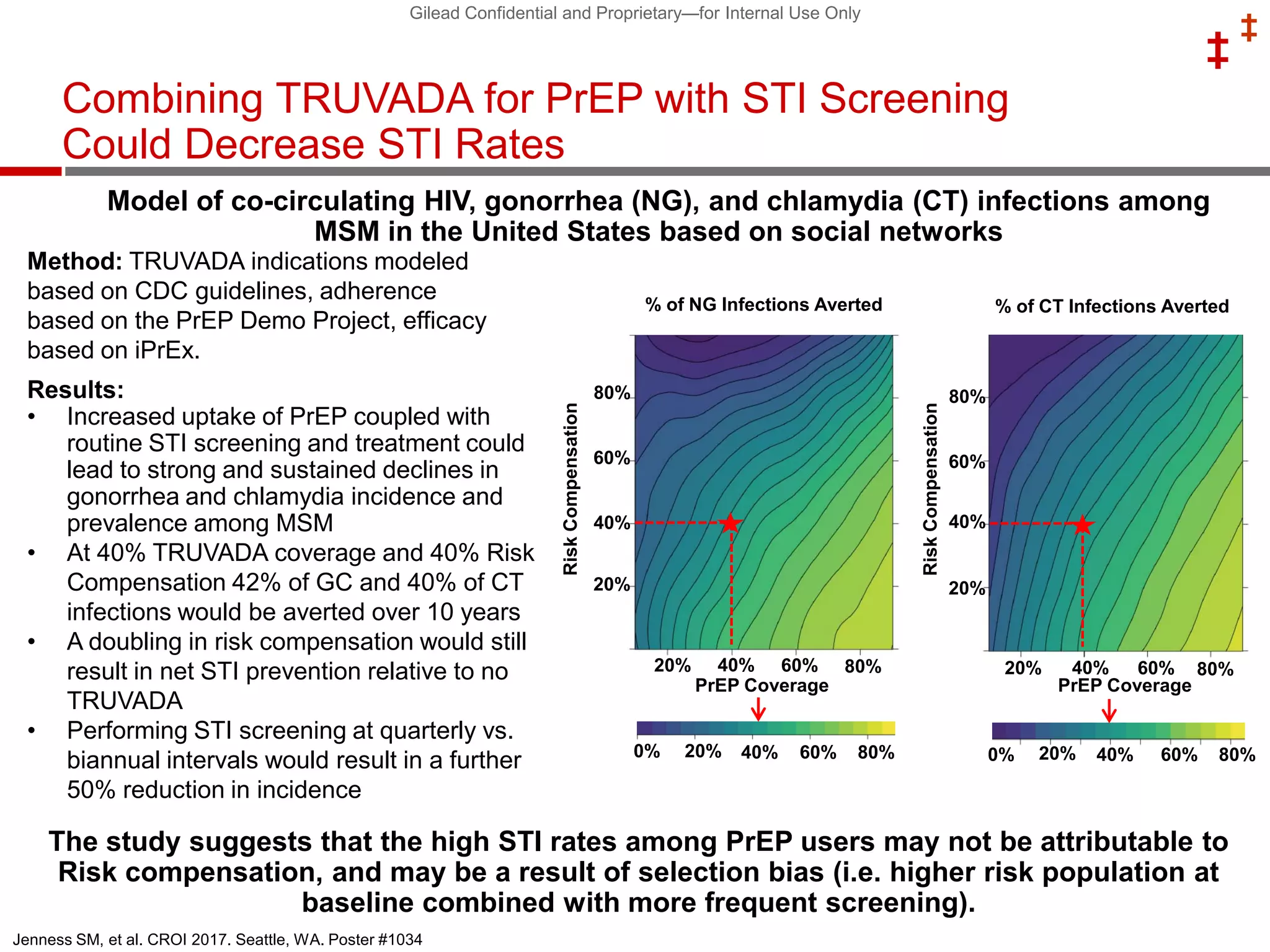
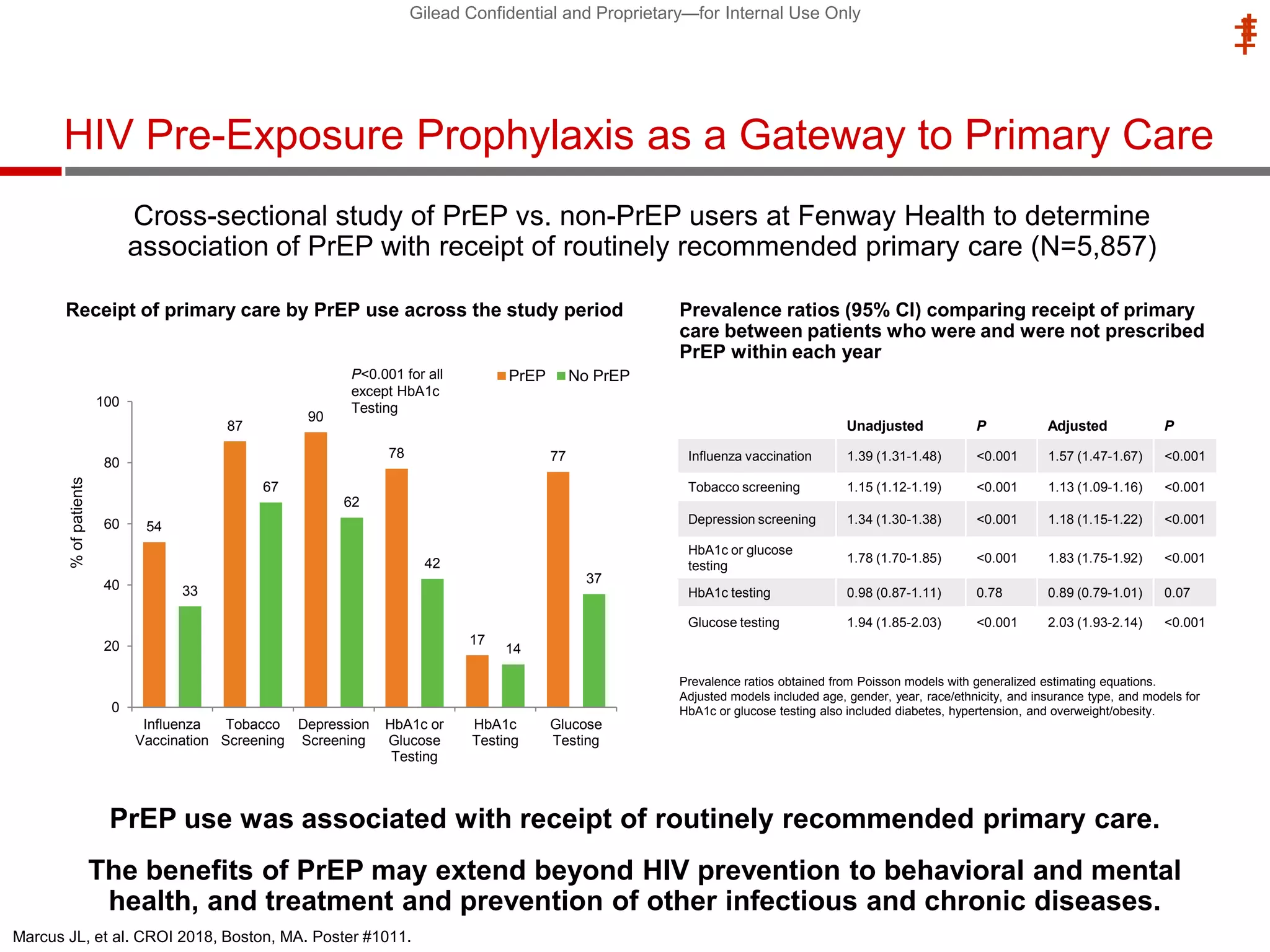
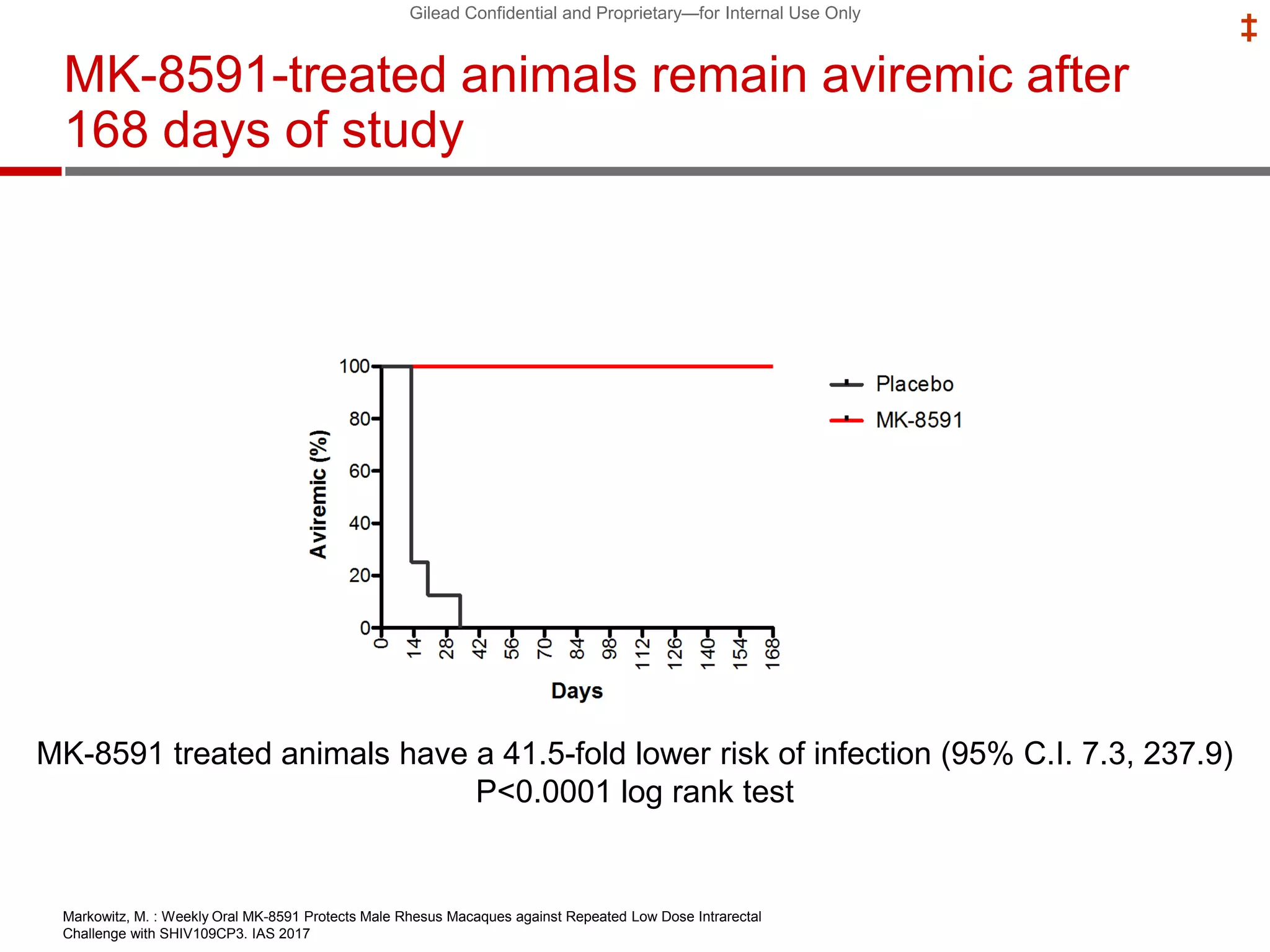
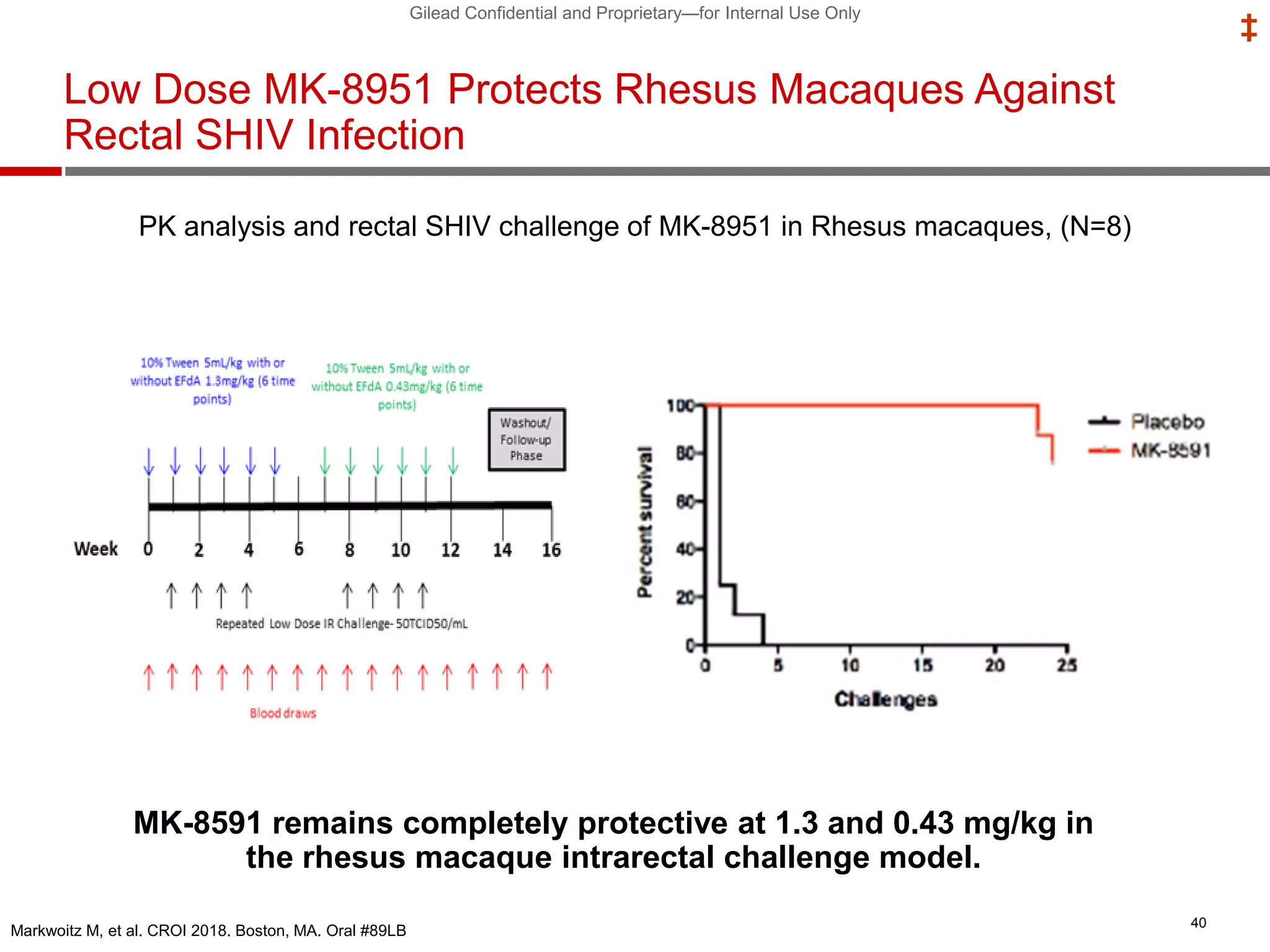
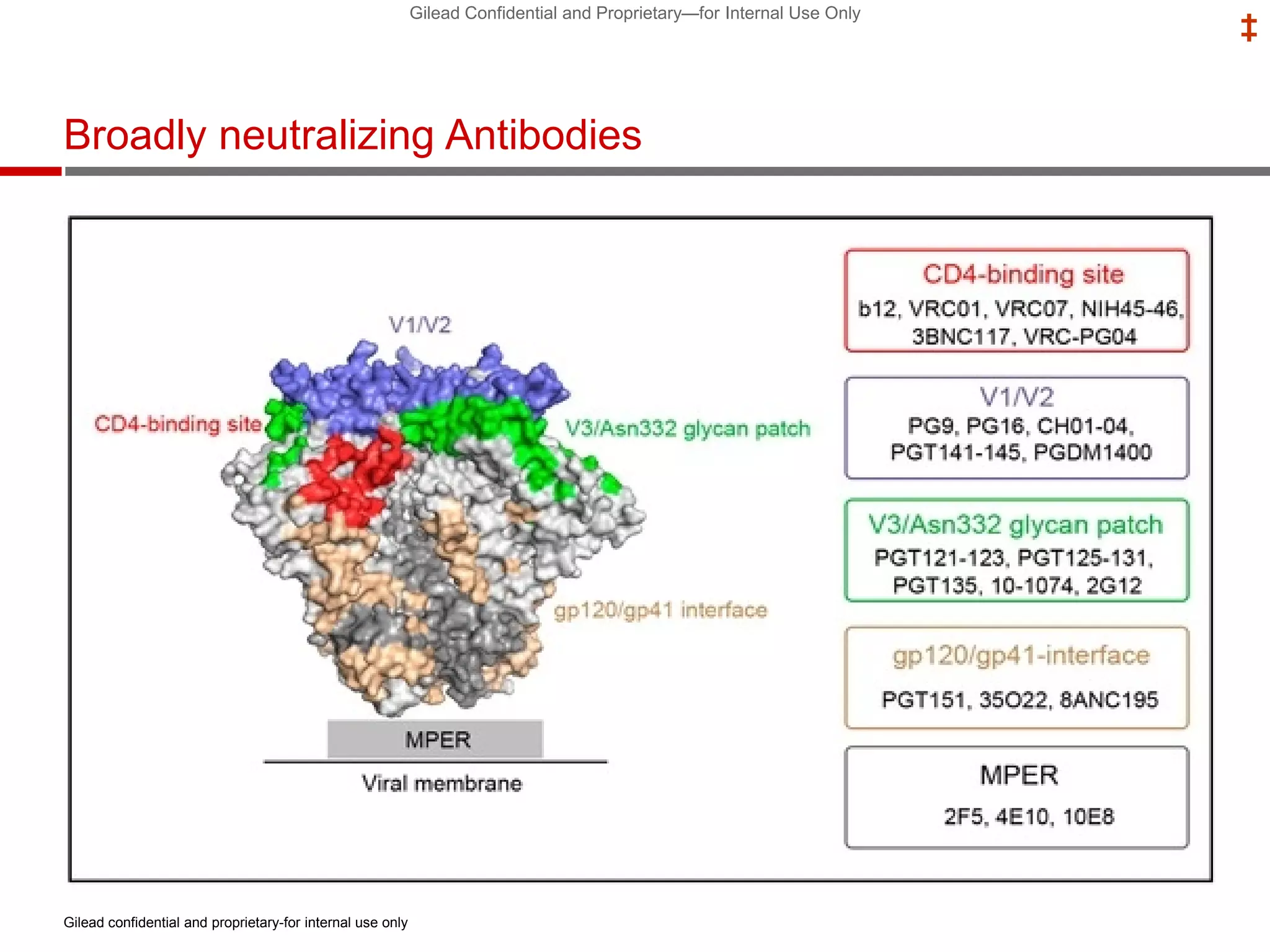
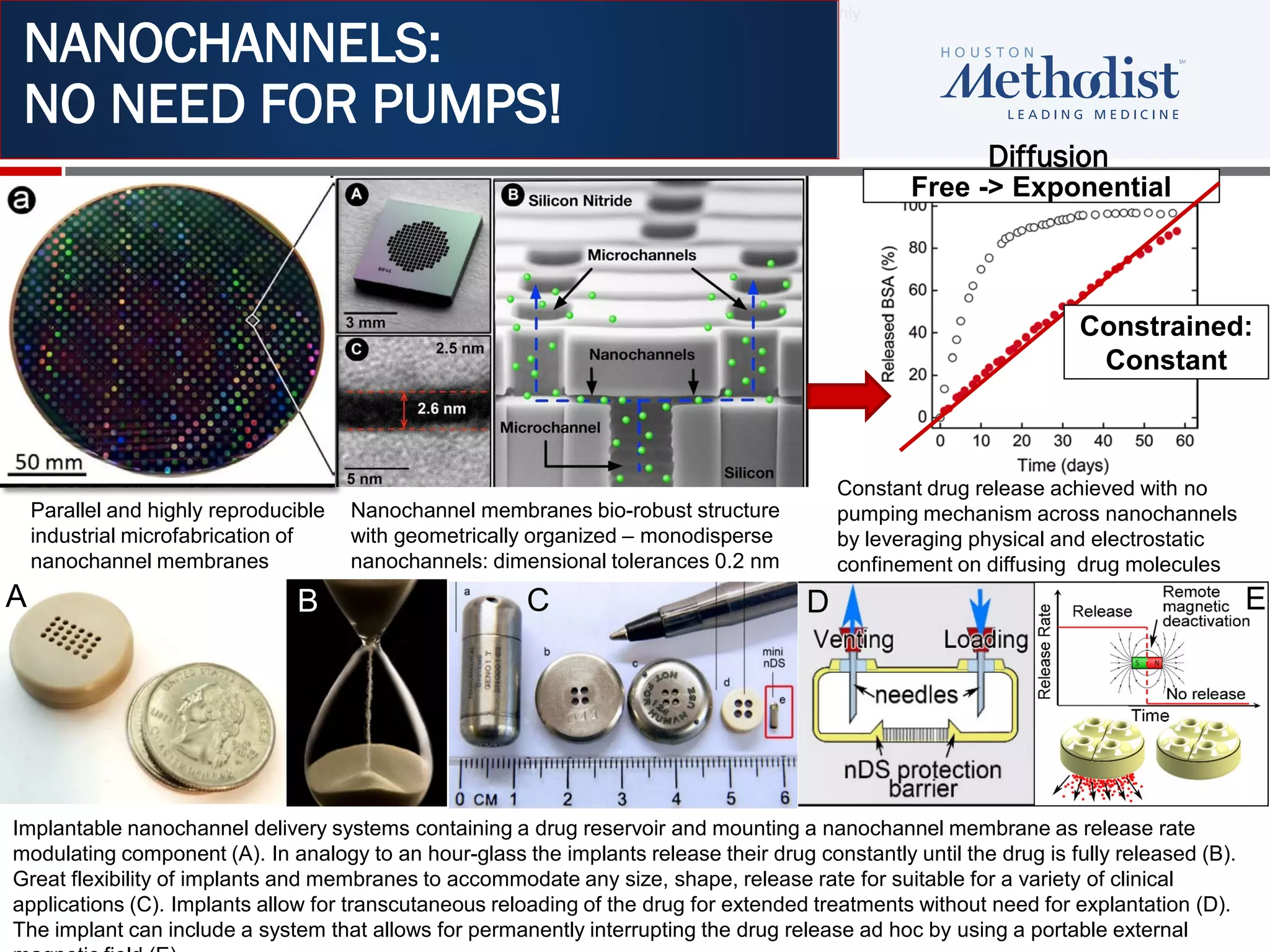
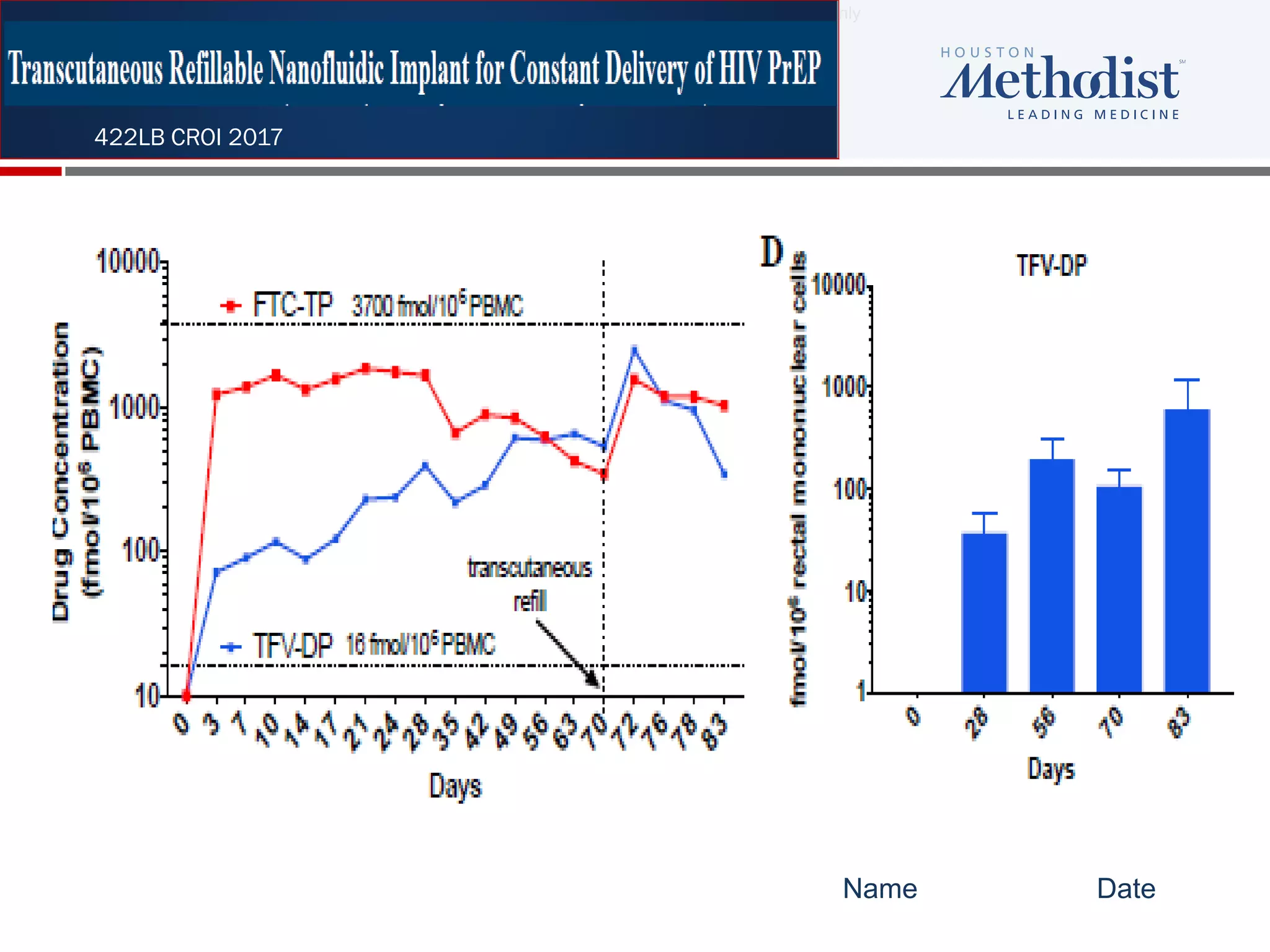
This document summarizes information presented at the 2018 Conference on Retroviruses and Opportunistic Infections (CROI) regarding pre-exposure prophylaxis (PrEP) use and HIV prevention. Key points include:
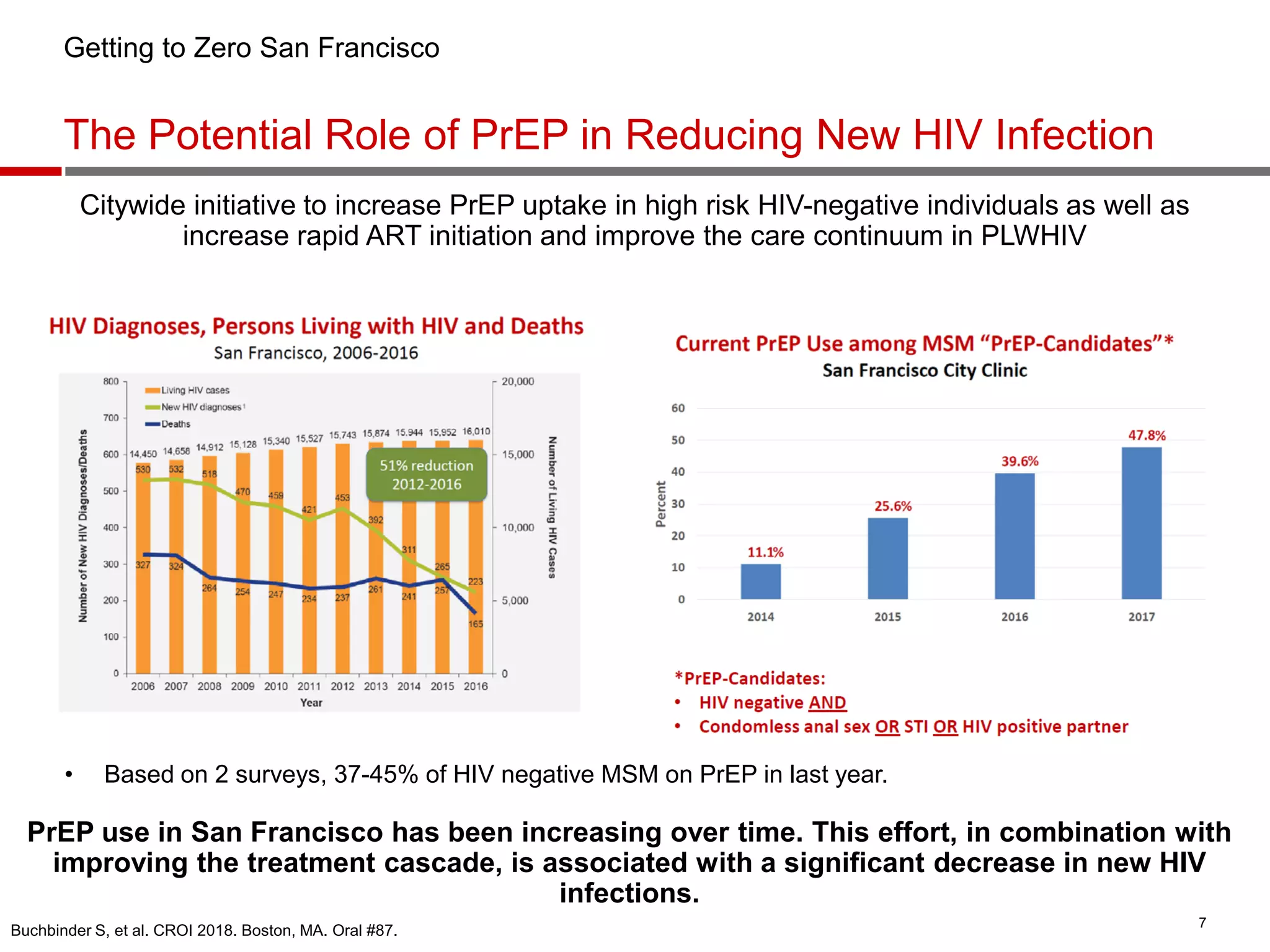
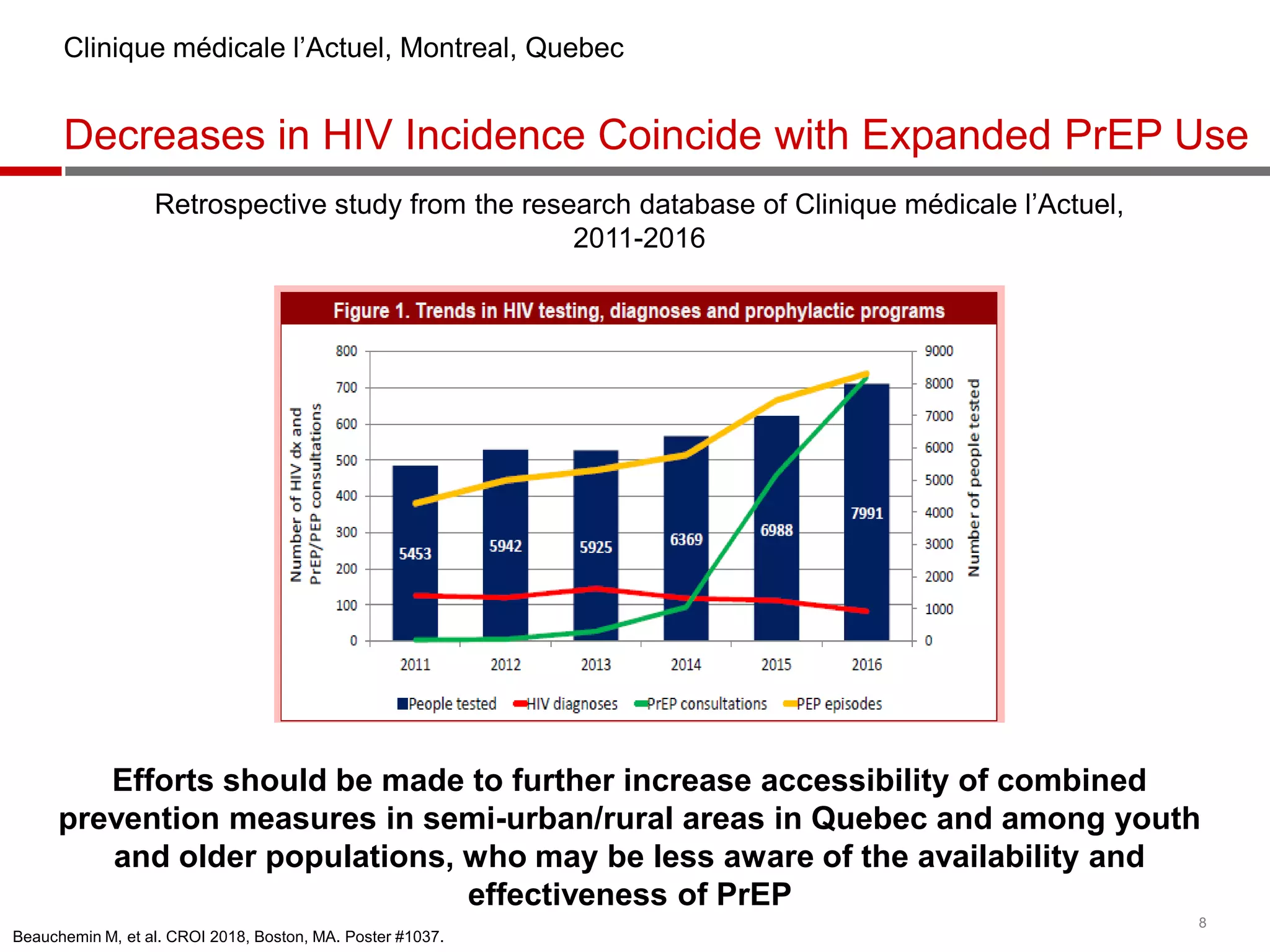
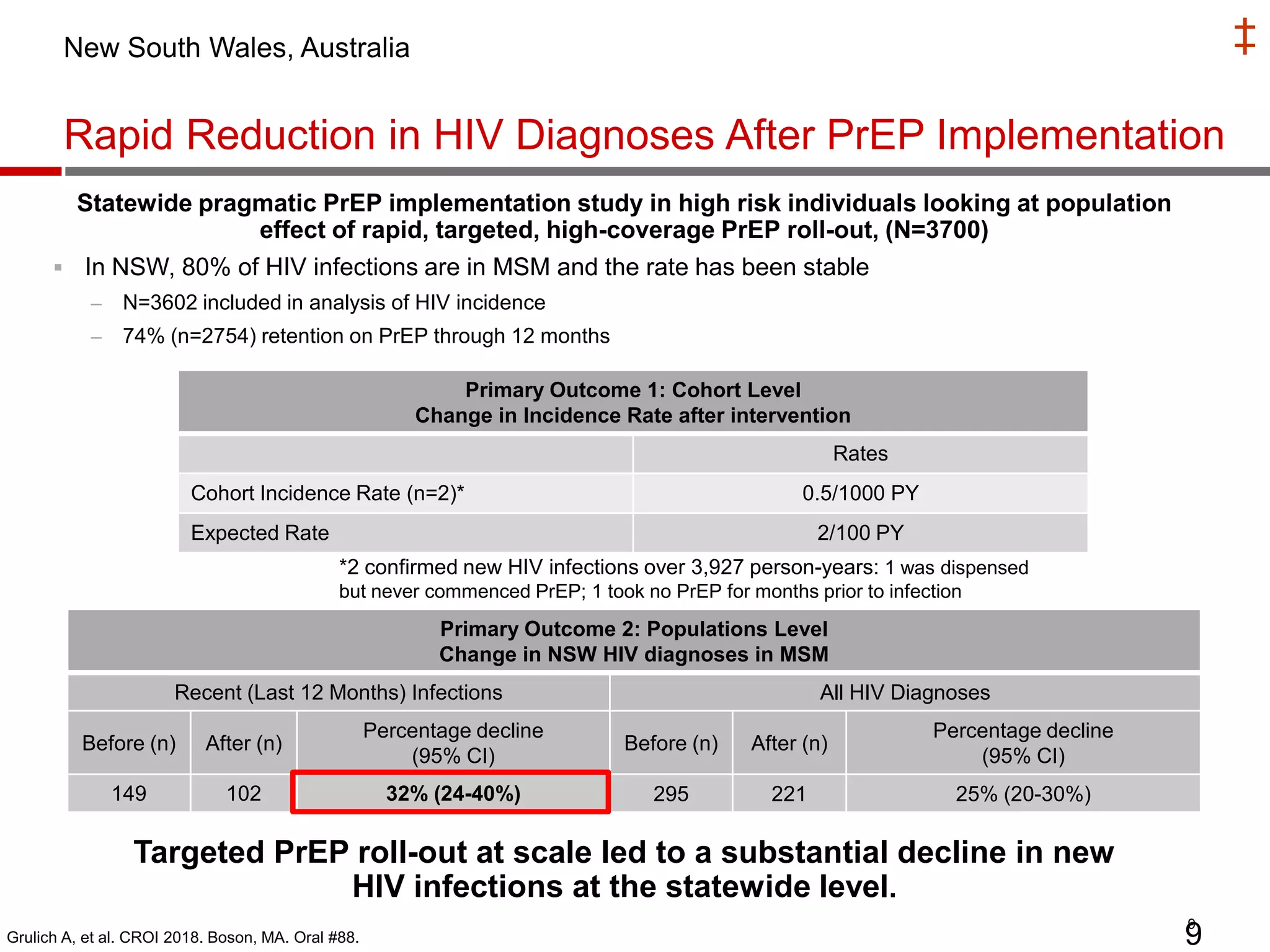
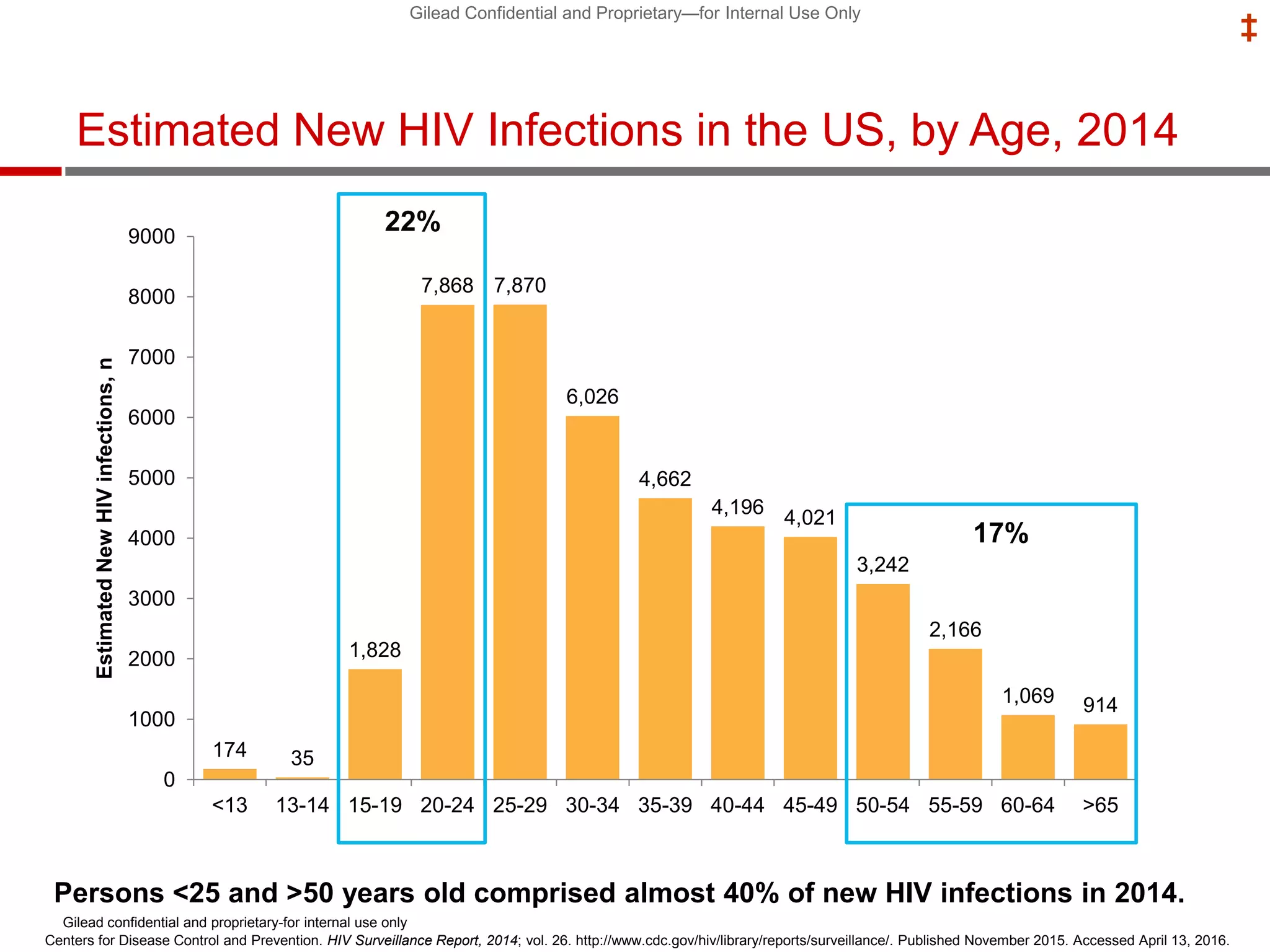
- PrEP use has increased HIV prevention efforts but more is needed to reach global prevention targets. Studies showed declines in new HIV diagnoses associated with PrEP scale-up in San Francisco, Australia, and Montreal.
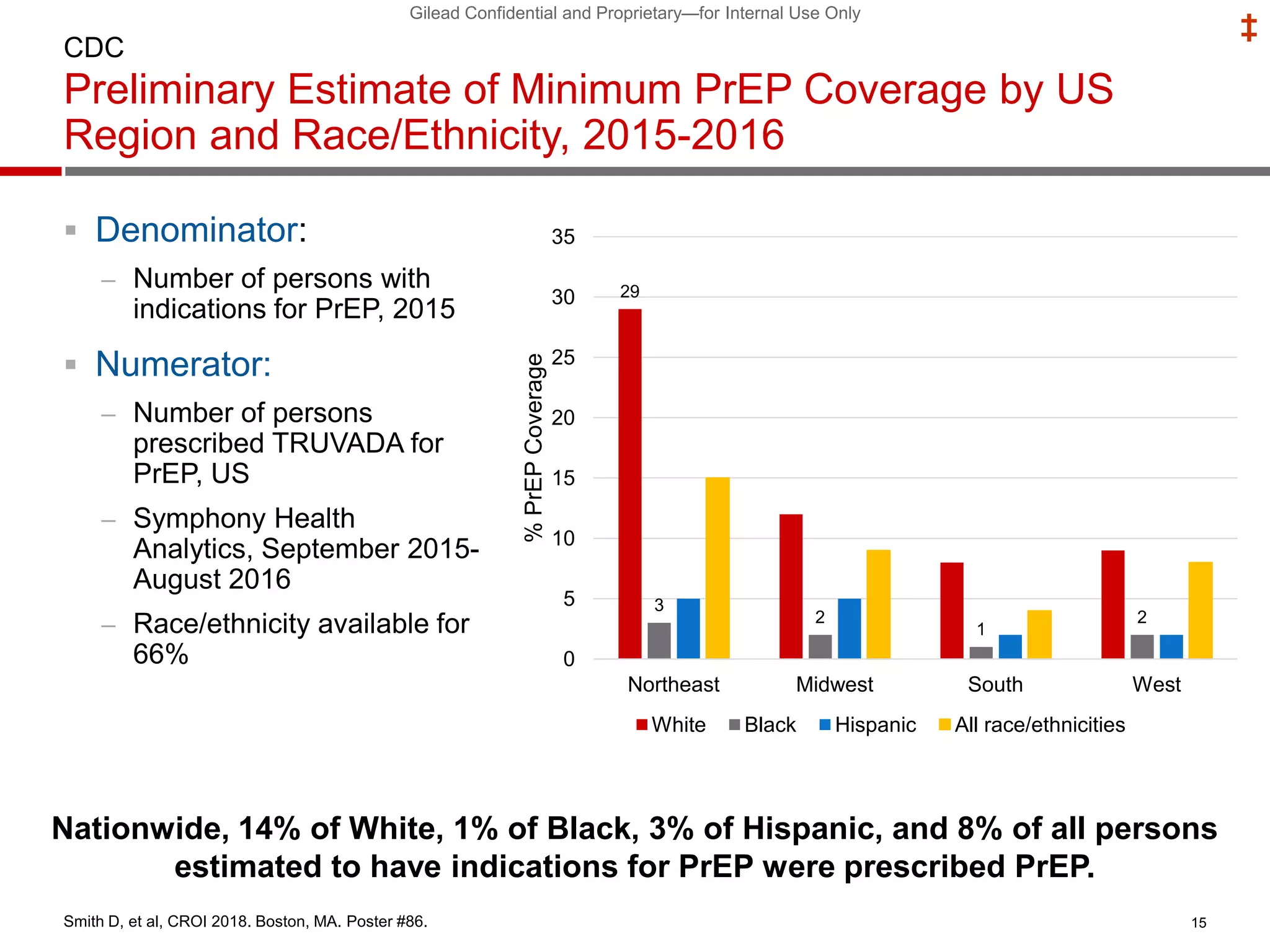
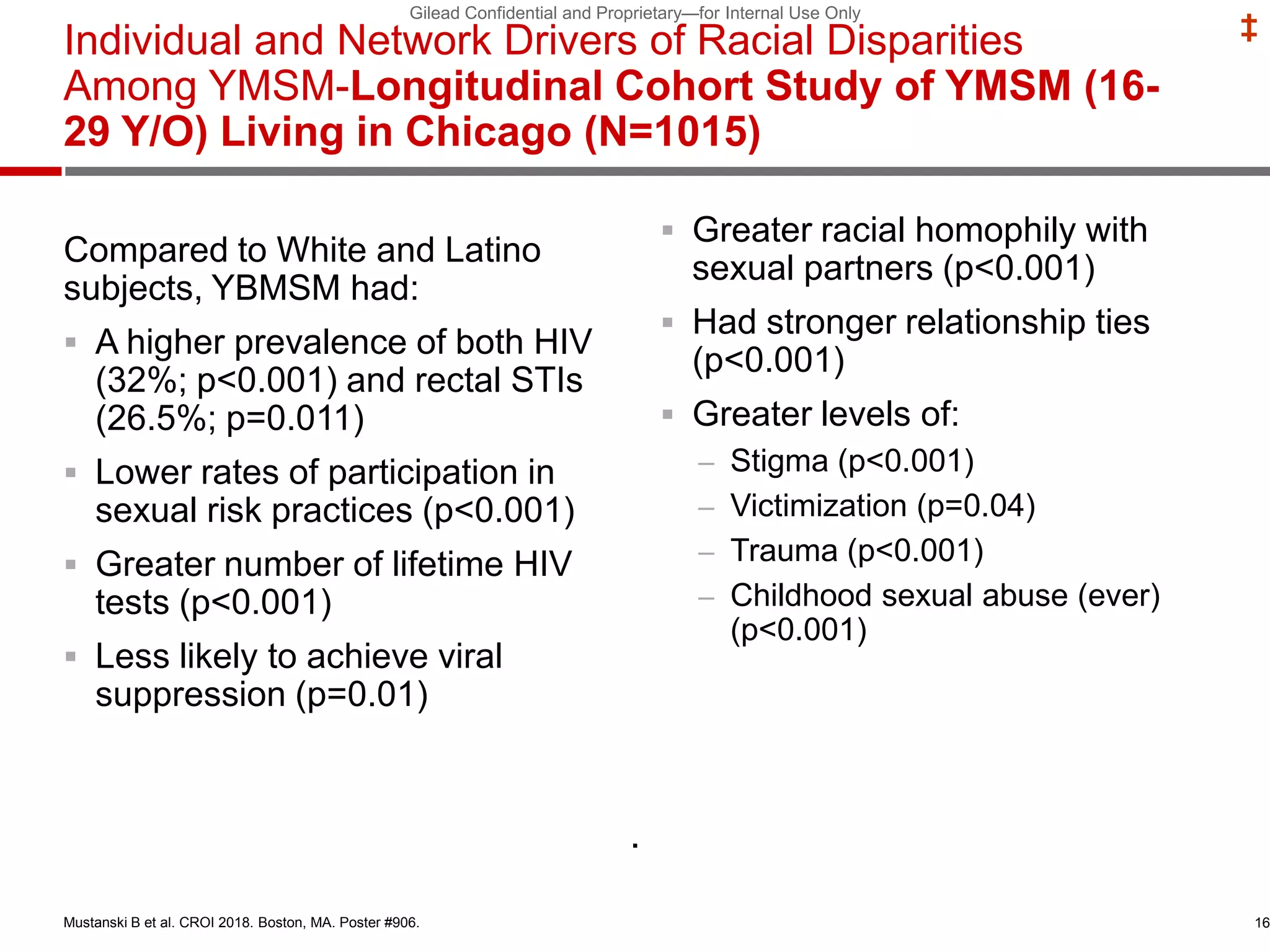
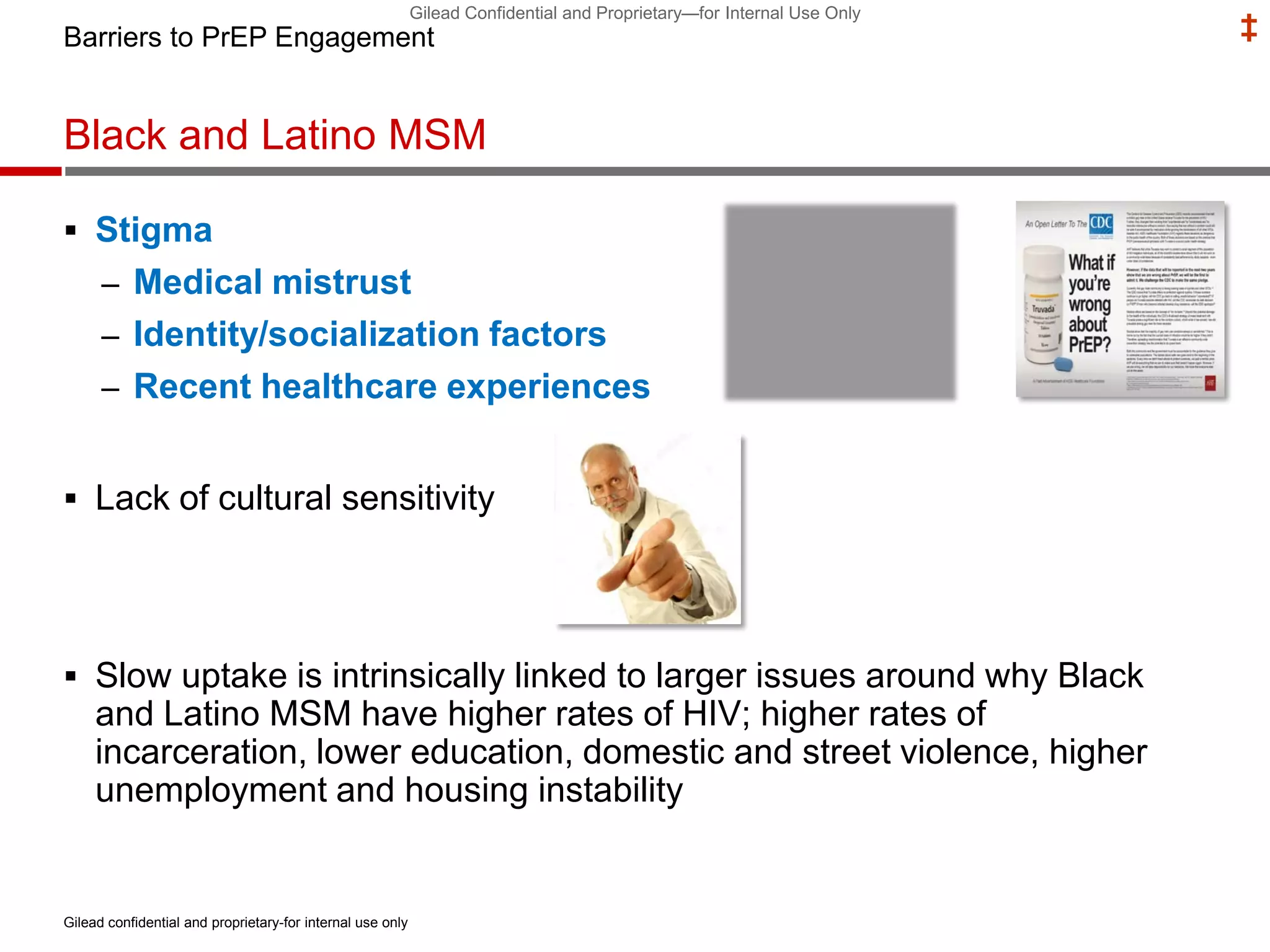
- Uptake of PrEP remains low among populations most at risk like Black and Hispanic men who have sex with men. Estimates found only 1% of Black individuals and 3% of Hispanic individuals with PrEP indications were prescribed PrEP.
- Barriers to