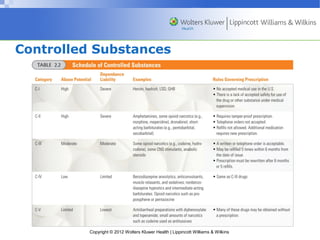
This document discusses the development, regulation, and delivery of pharmaceutical drugs. It covers the sources of drugs, including plants, animals, synthetic chemicals, and genetically engineered chemicals. The document then discusses the drug development process, including preclinical and clinical trials, as well as the various laws and regulations governing drug advertising, scheduling of controlled substances, and distribution. It emphasizes the important role of nurses in properly managing drug therapy within the legal and institutional controls.