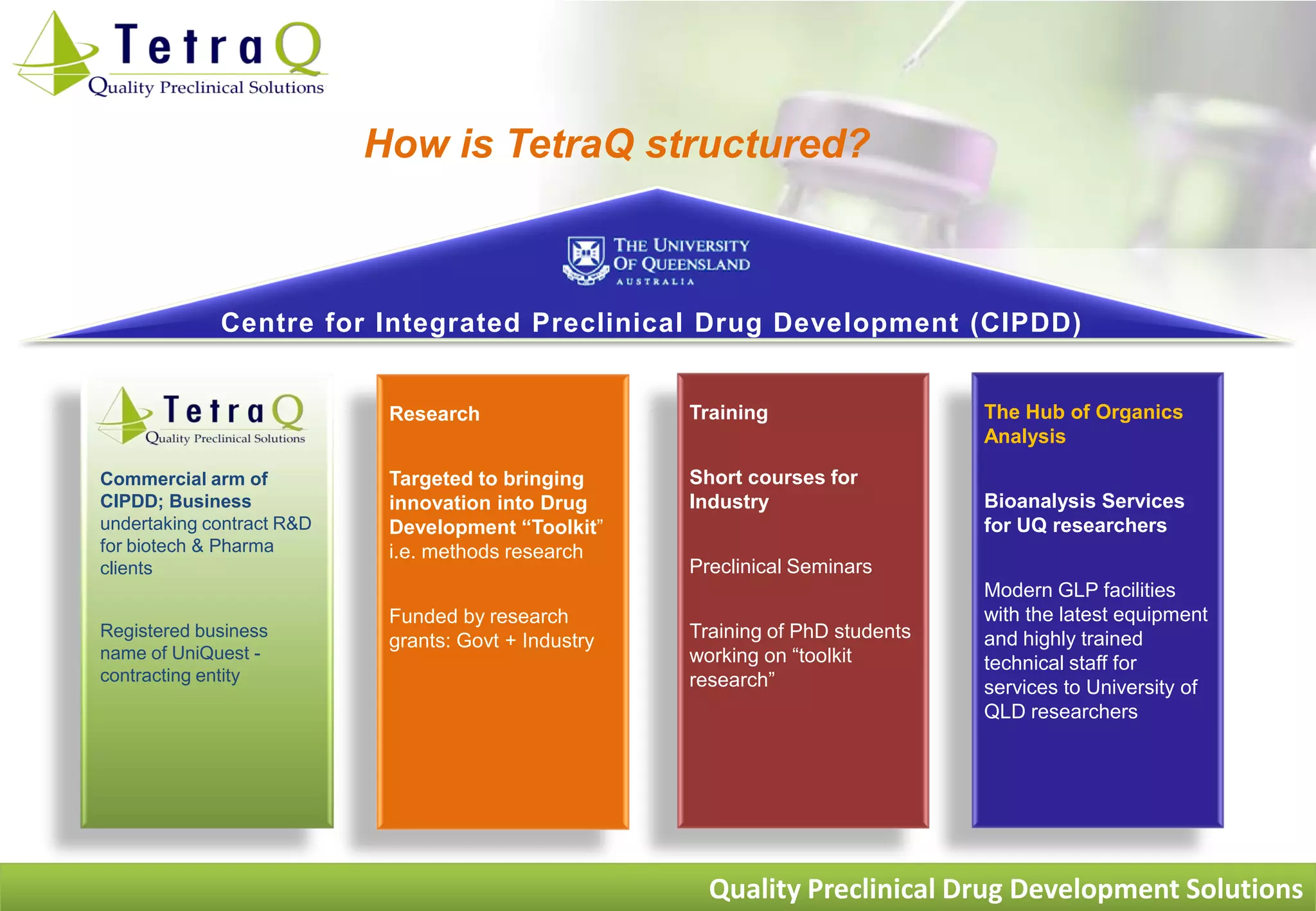
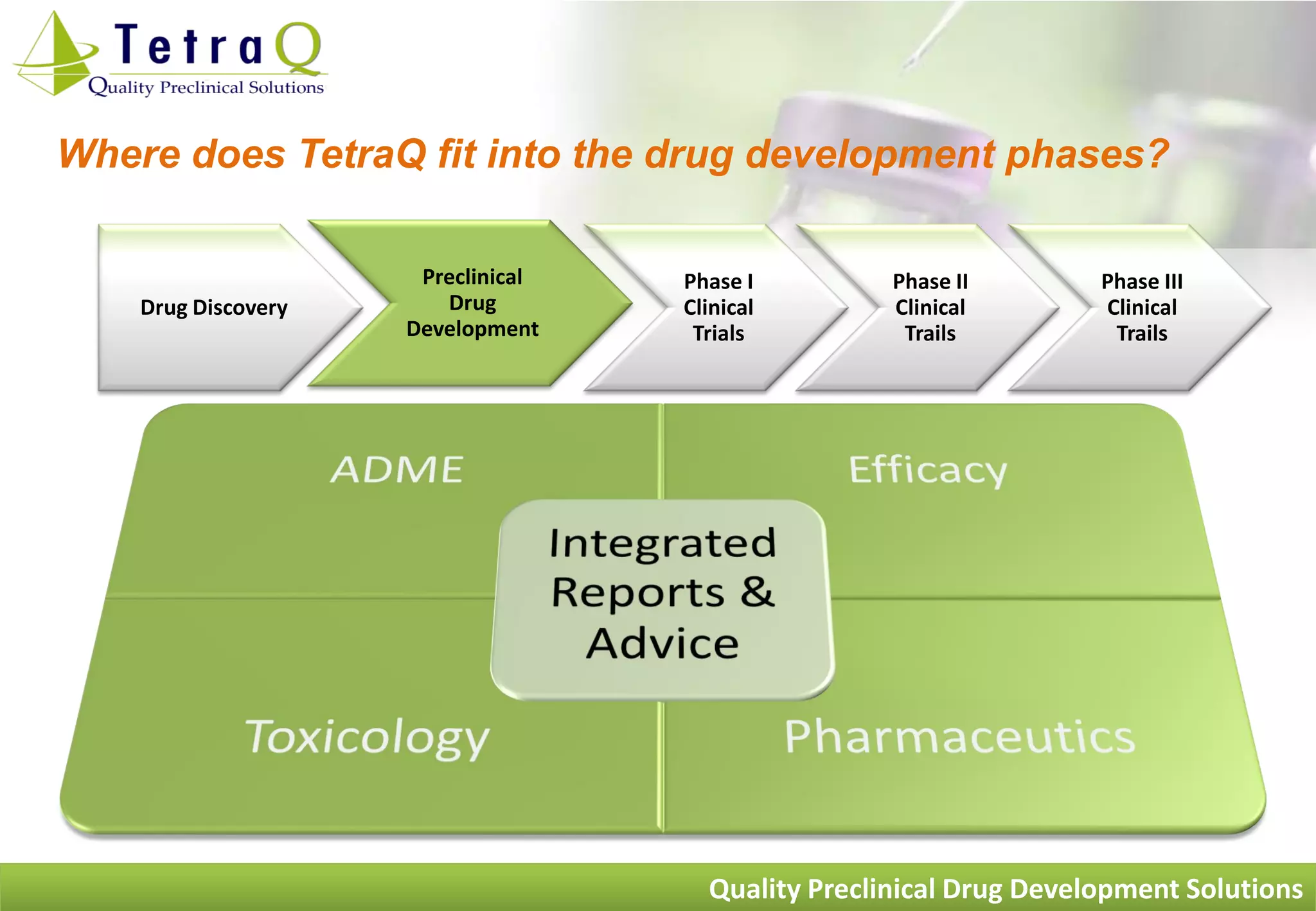
TetraQ is a preclinical drug development facility located at the University of Queensland in Brisbane, Australia. It was founded in 2005 by four experts in the core areas of preclinical drug development - efficacy, ADME, toxicology, and pharmaceutics. TetraQ provides a range of preclinical services including efficacy testing in disease models, ADME/pharmacokinetic studies, toxicology testing, and formulation development to assess drug candidates and help reduce risks prior to clinical trials. The services help determine whether drug candidates are likely to be safe and effective enough to advance to human studies.