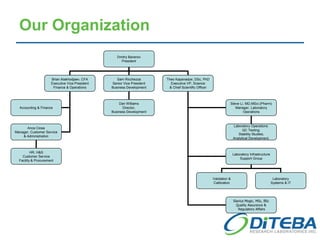
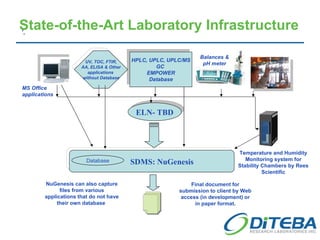
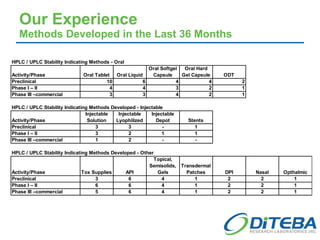
Diteba is a cGMP/GLP pharmaceutical laboratory located near Toronto, Canada that offers analytical R&D services including method development and validation, bioanalytical testing, quality control release testing, stability testing, and in vitro release testing. The company has over 10,000 square feet of laboratory space equipped with state-of-the-art instrumentation. Diteba's team of senior scientists have extensive experience across various therapeutic areas and analytical techniques.