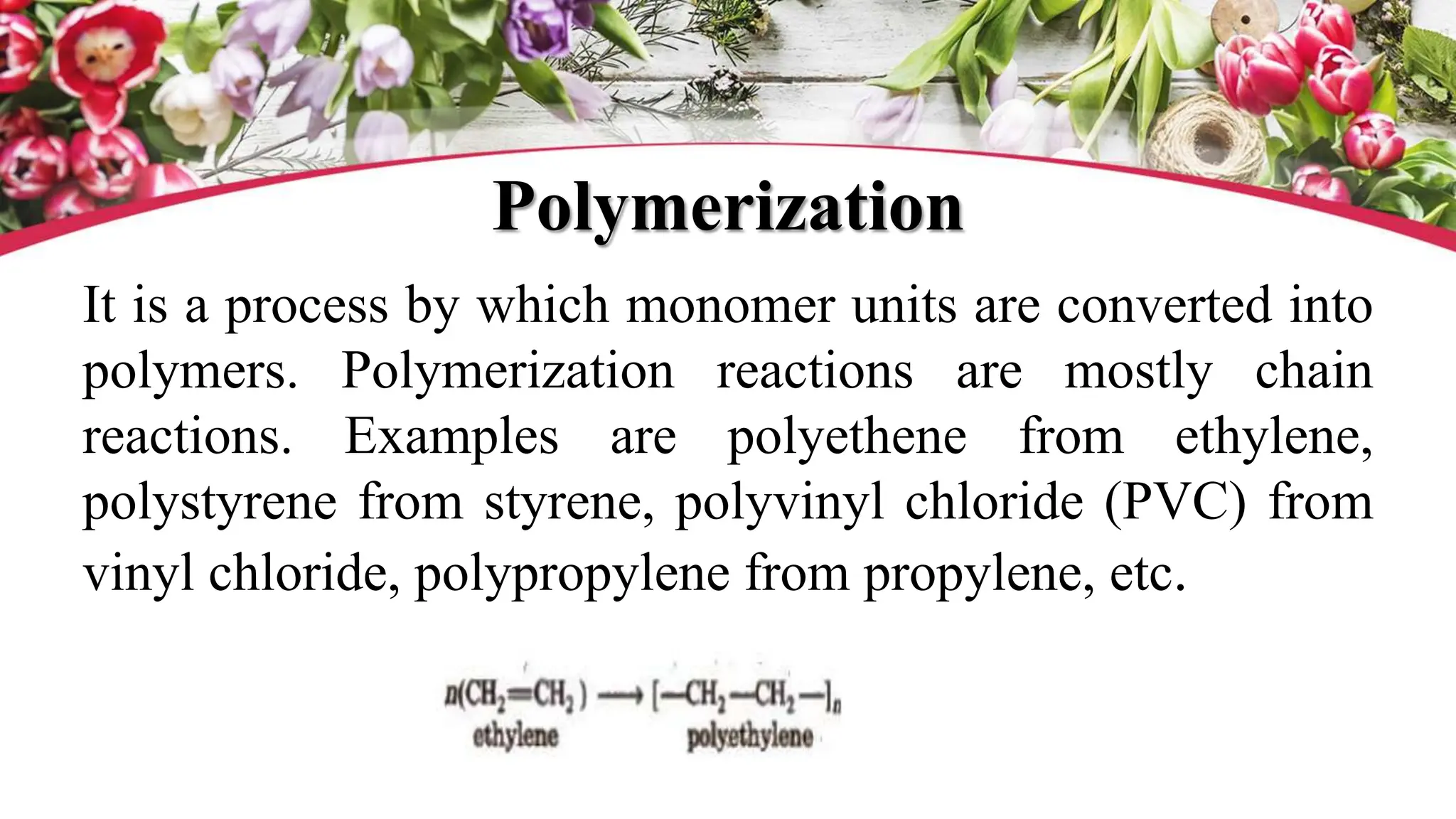
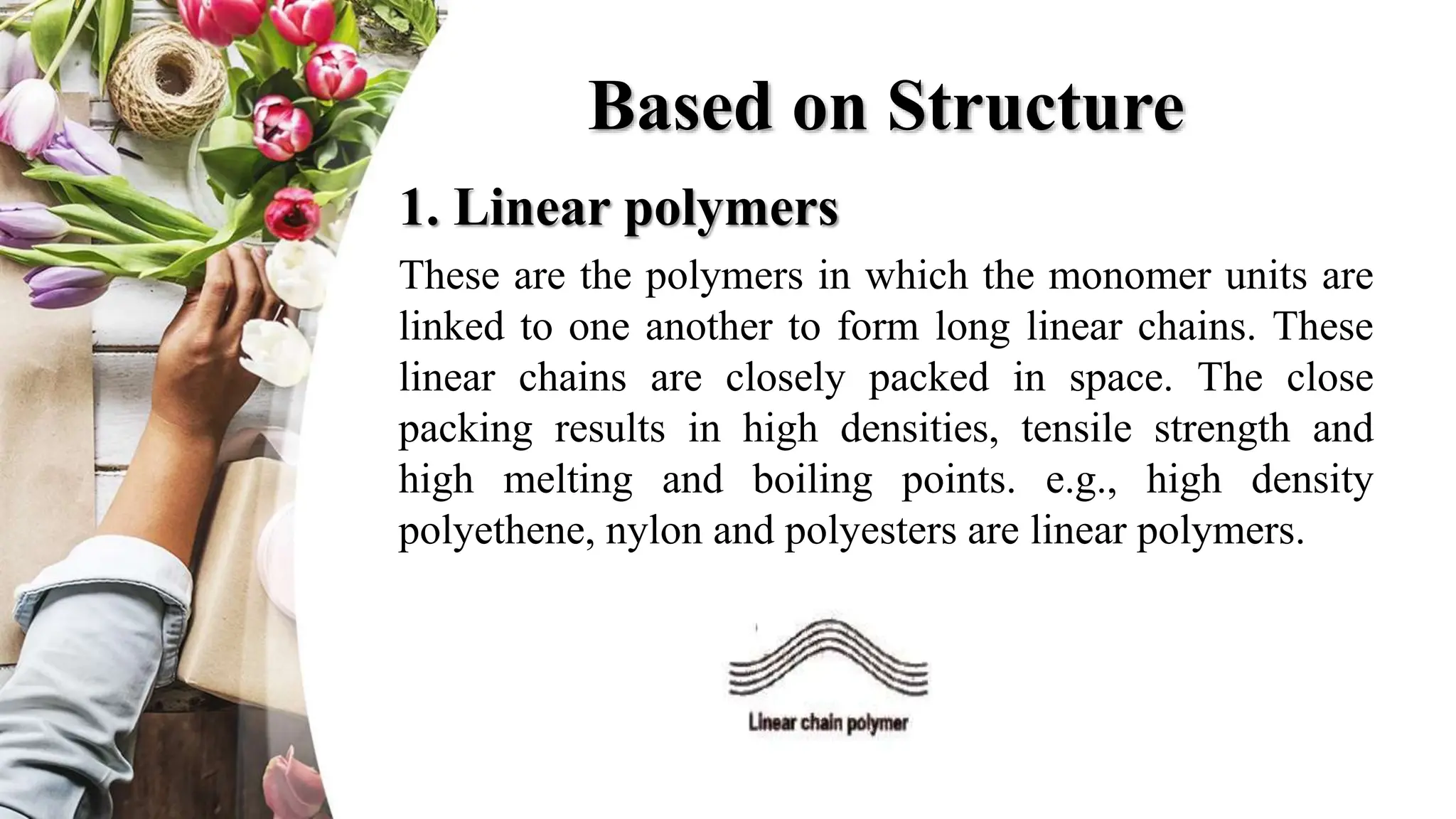
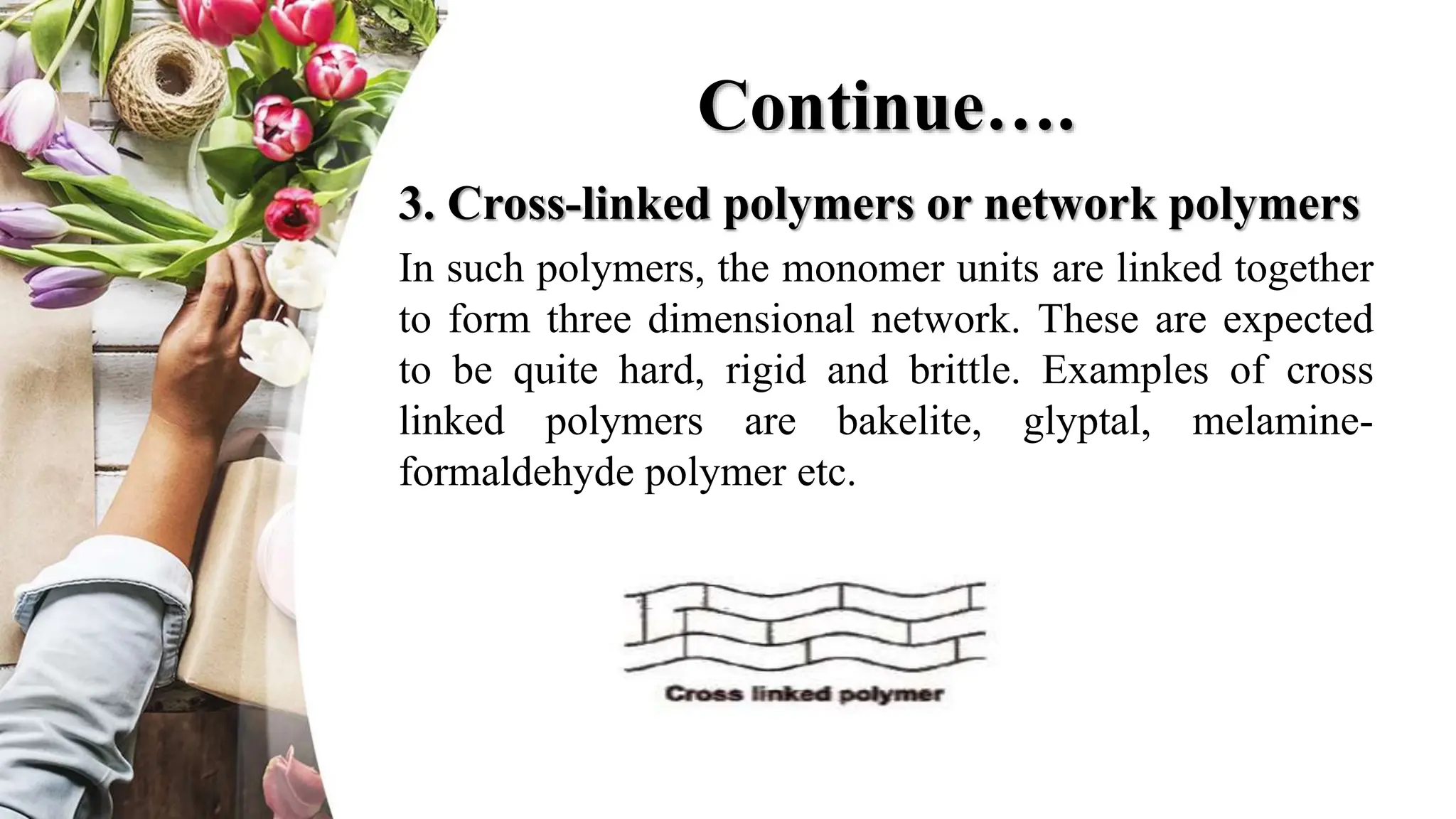
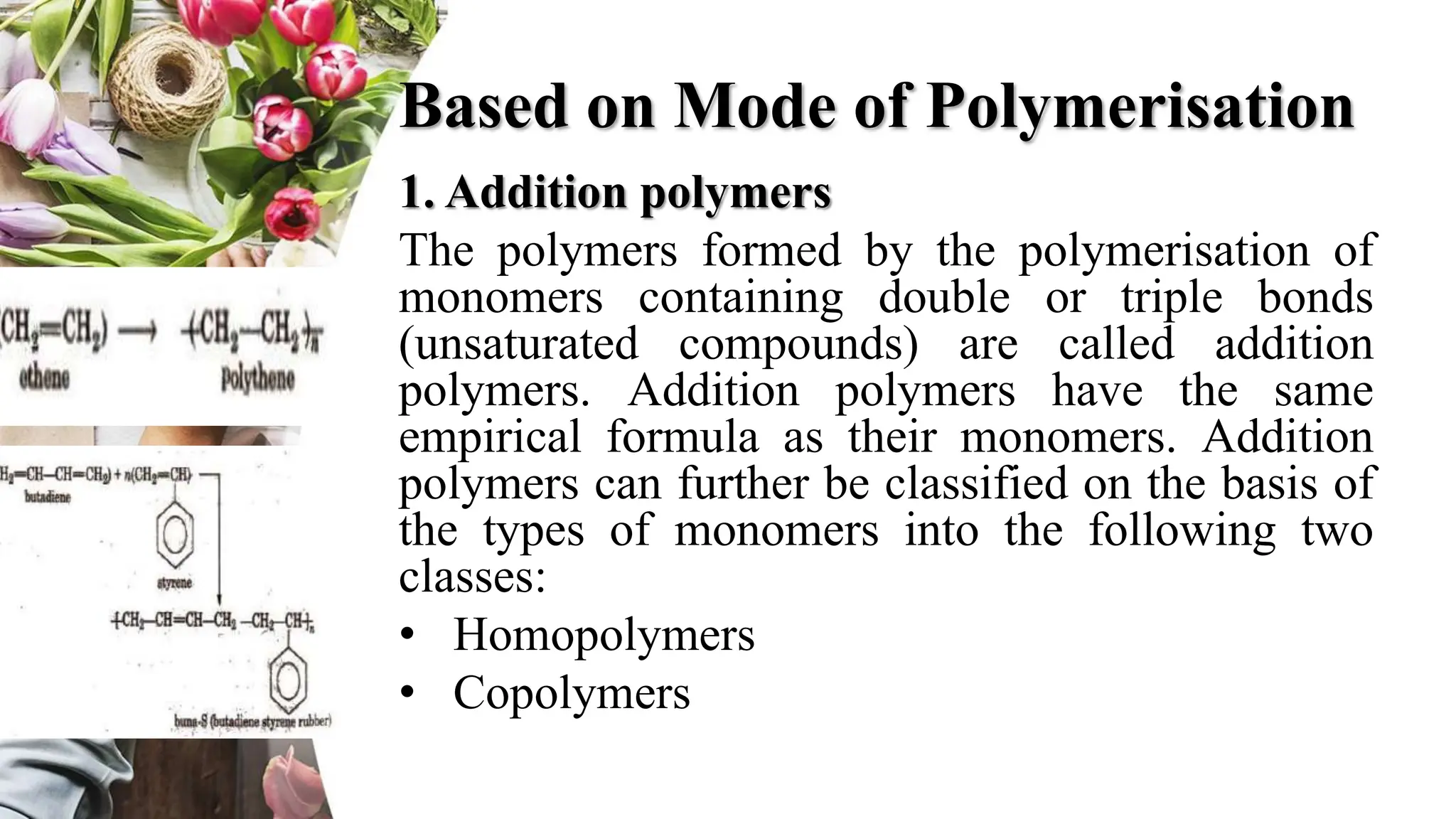
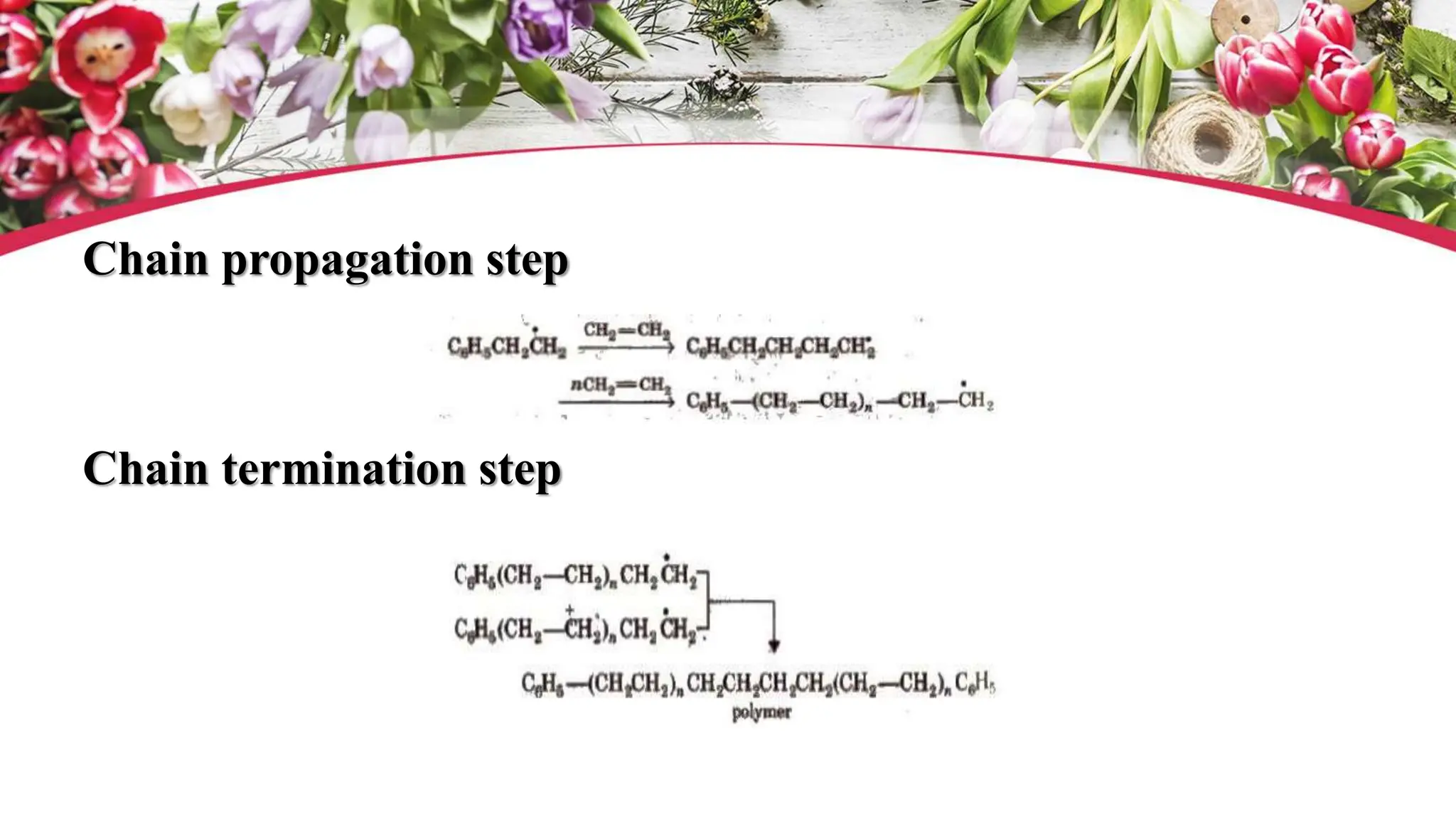
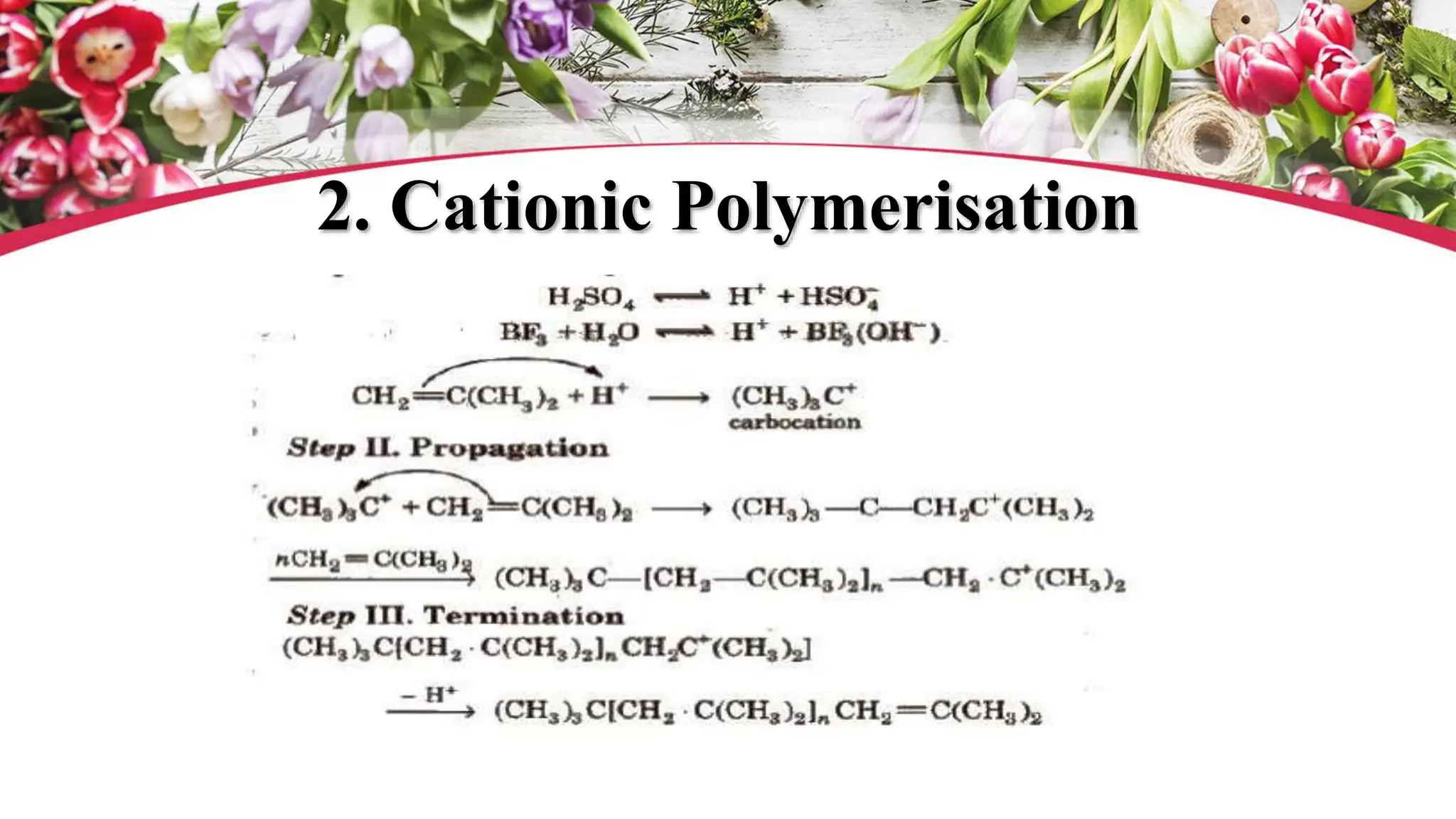
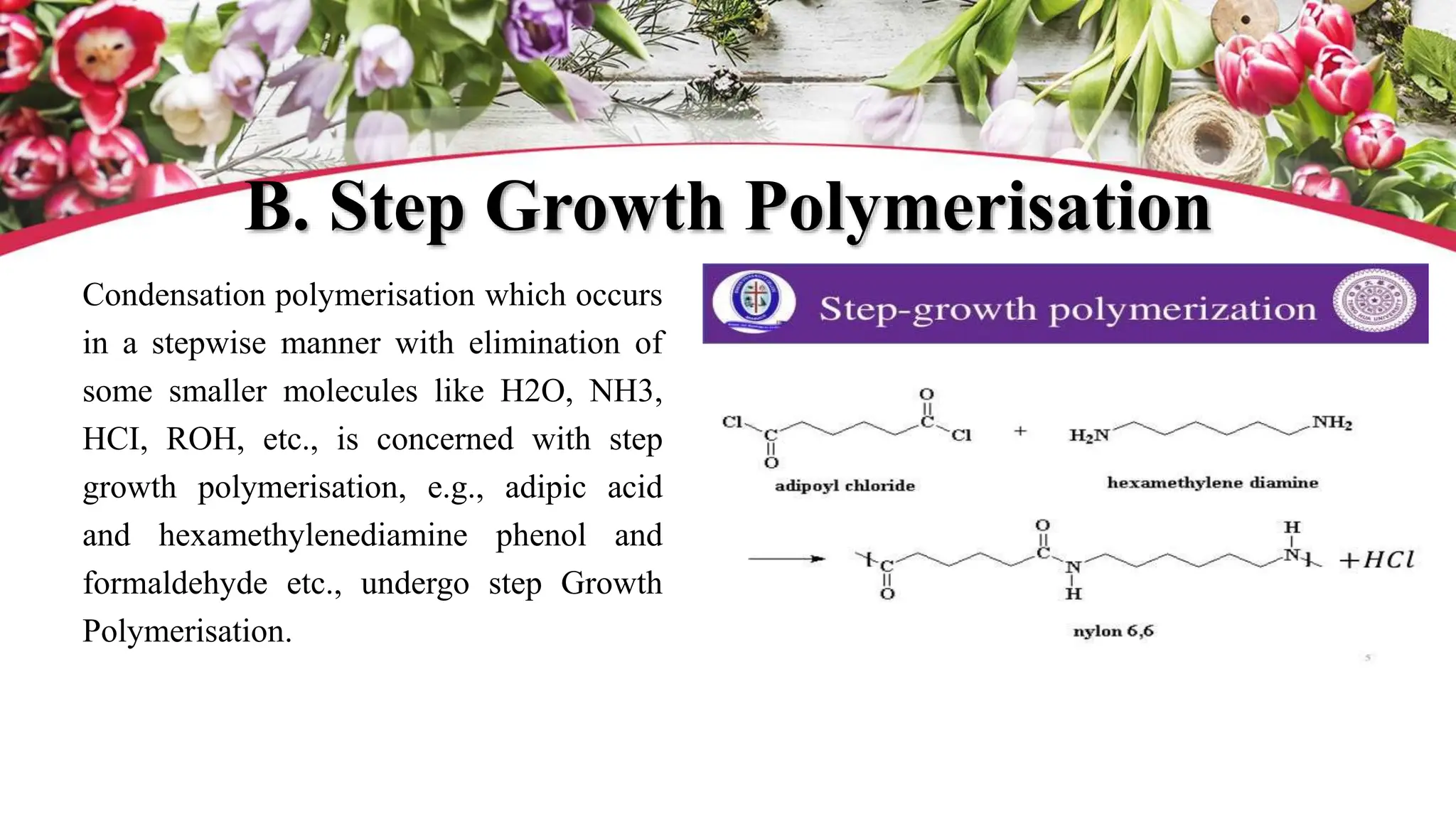
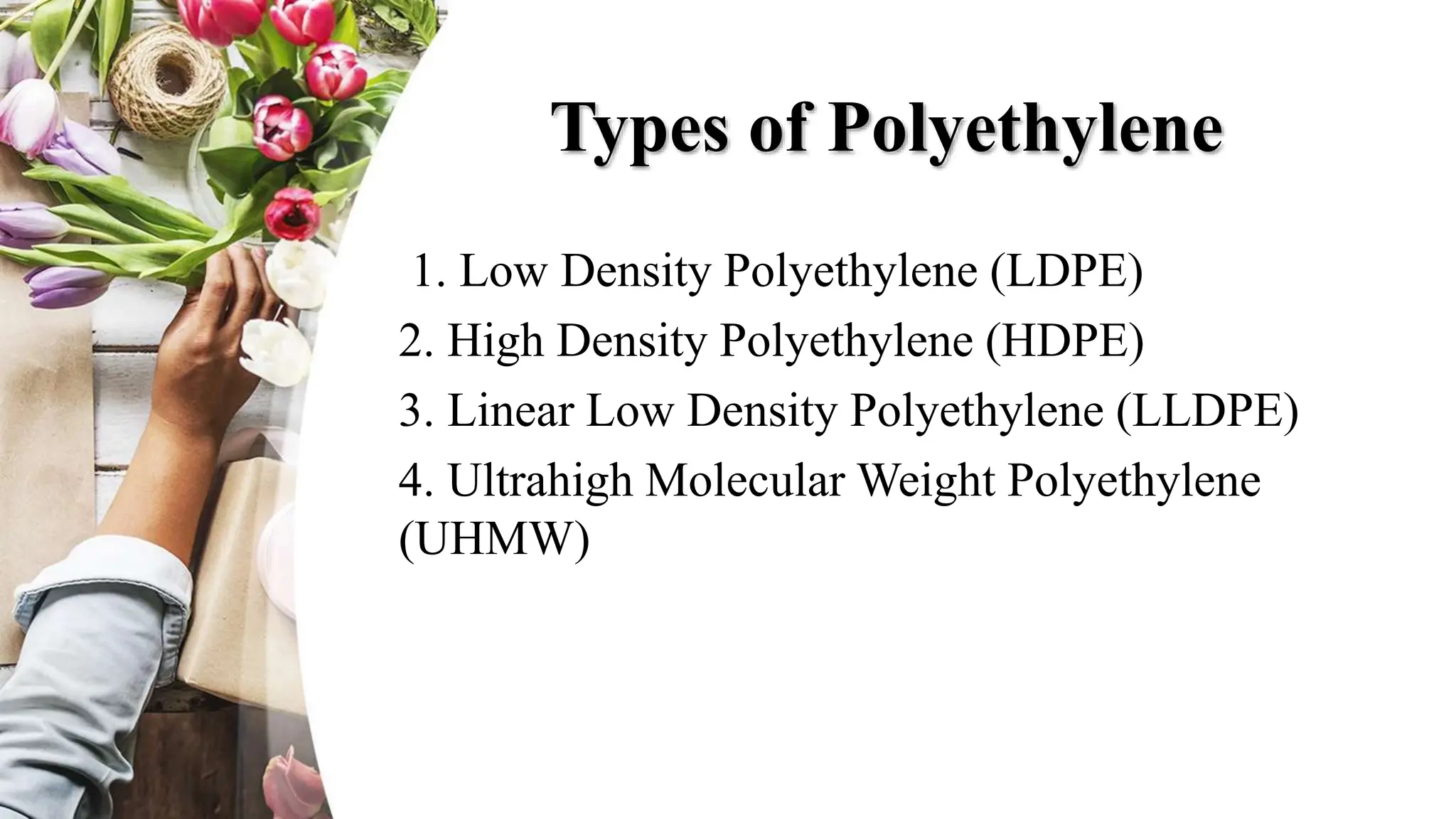
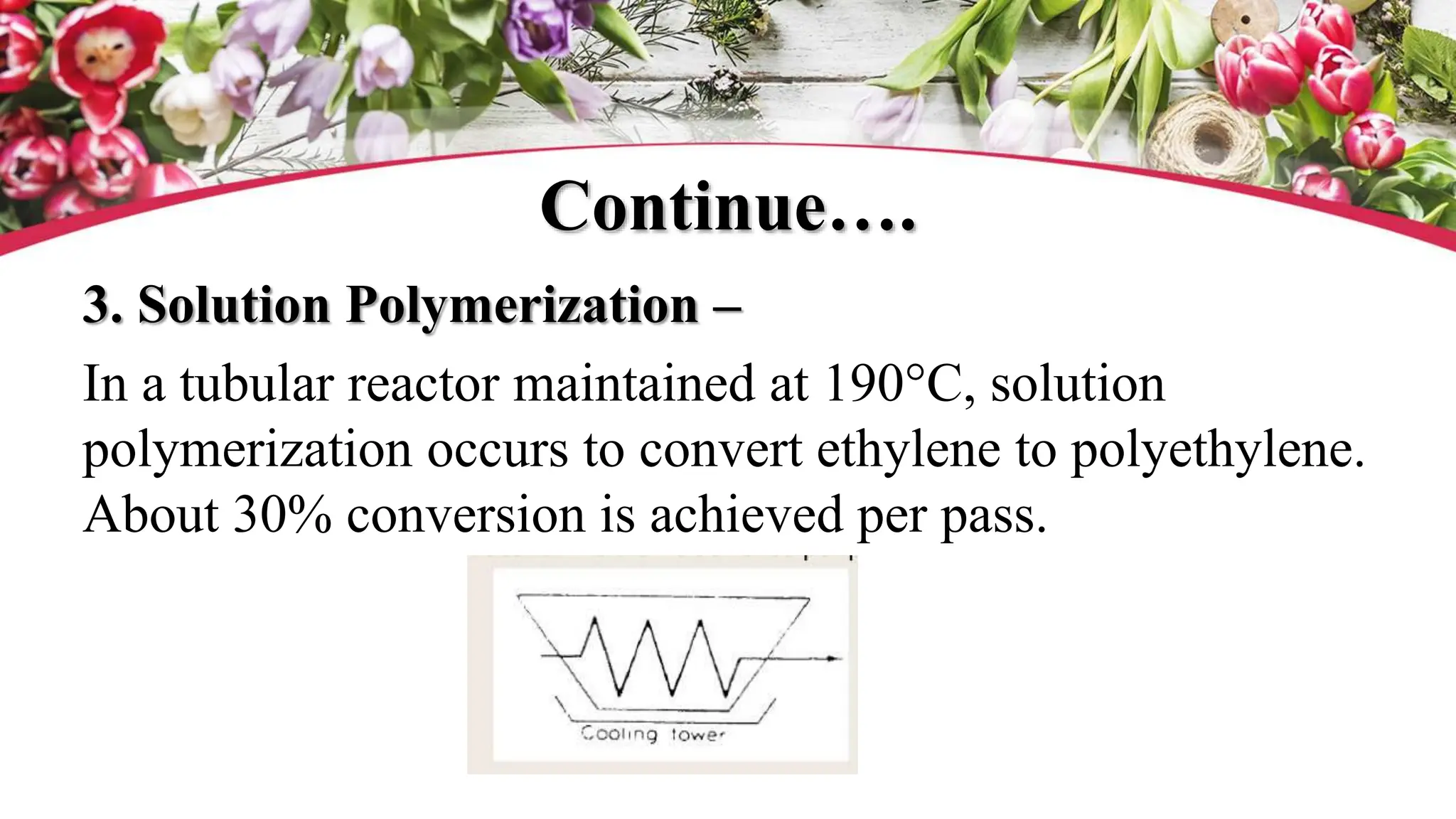
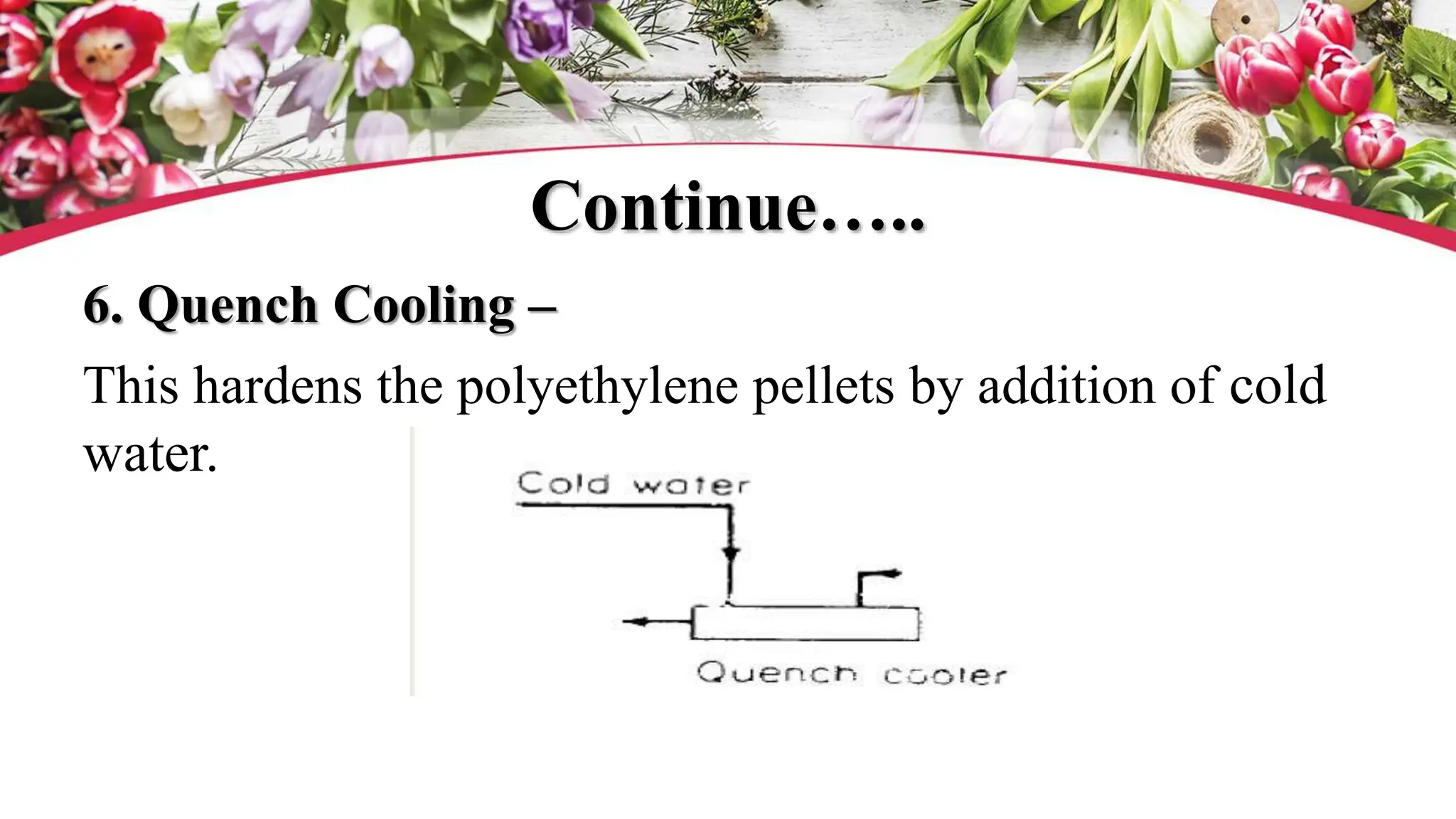
The document discusses polyethylene polymerization, defining polymers and their types, along with various classifications based on origin, structure, and polymerization methods. It details the production processes for low-density polyethylene (LDPE) and distinguishes between types of polyethylene such as high-density polyethylene (HDPE) and linear low-density polyethylene (LLDPE). Additionally, it outlines the applications of different polyethylene types in industries such as packaging and construction.