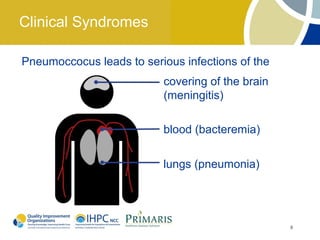
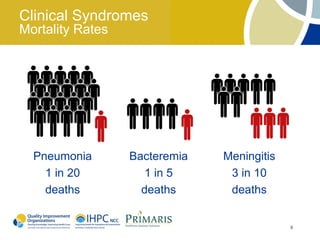
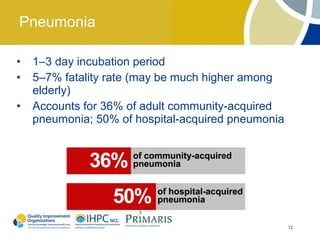
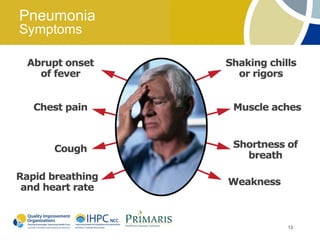
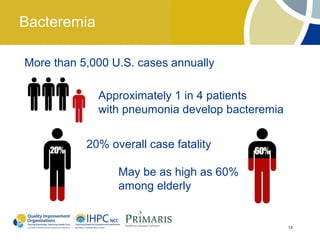
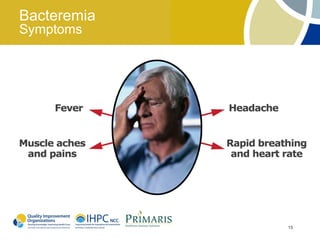
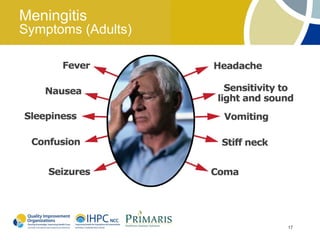
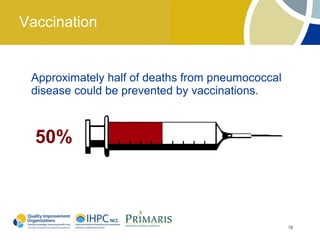
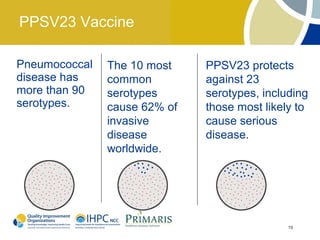
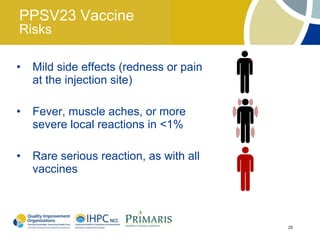
This document provides clinical guidelines for pneumococcal vaccination in older adults. It describes pneumococcal disease as a leading cause of vaccine-preventable illness and death in the US, especially dangerous for young children and adults aged 65 and older. It recommends vaccination with PPSV23 for adults aged 65 and older, as well as younger adults with certain medical conditions that increase risk. PPSV23 protects against the 23 serotypes known to cause the majority of invasive pneumococcal disease. While it is effective at preventing severe disease, it may not prevent all cases of pneumococcal pneumonia.