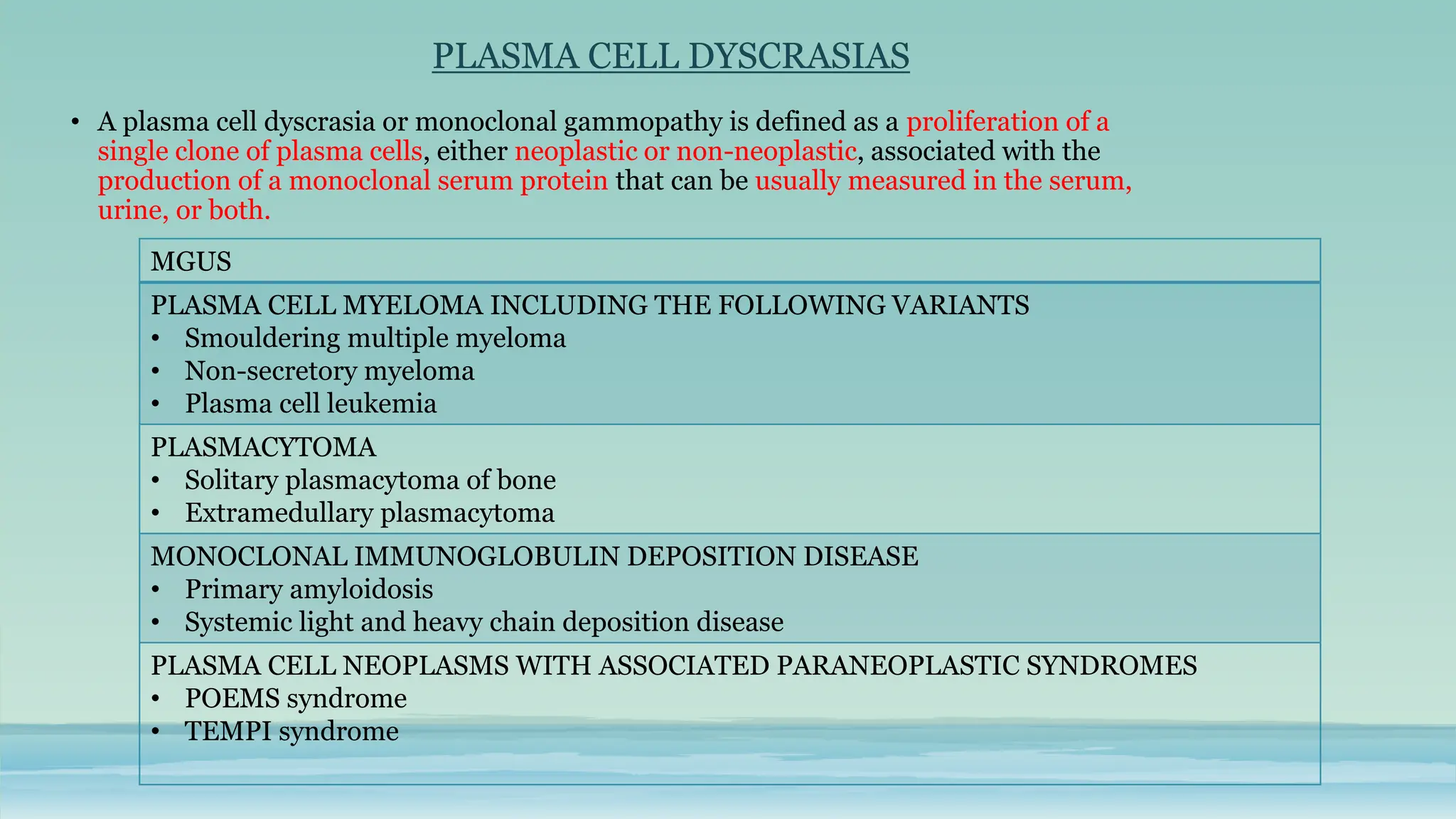
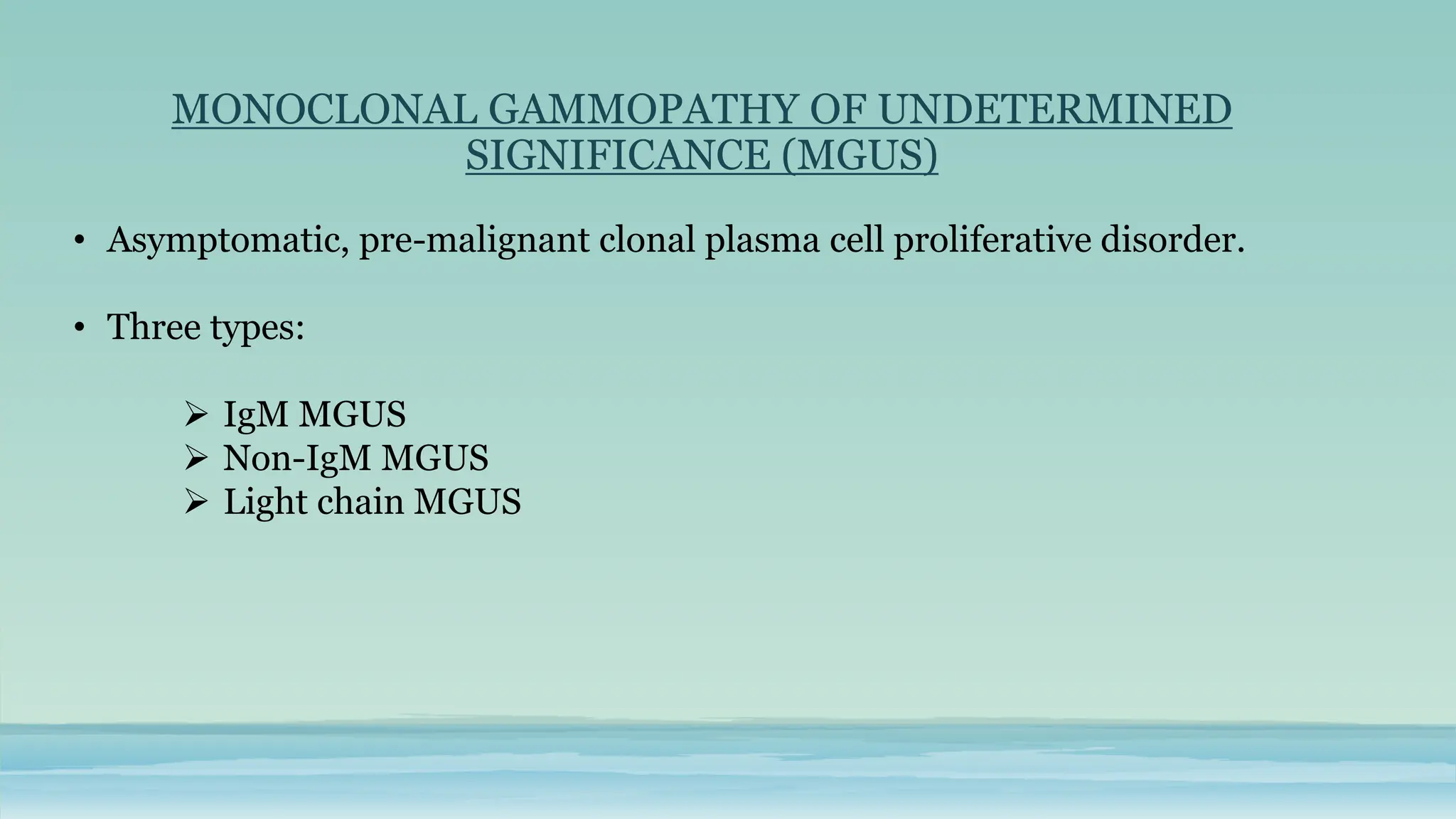
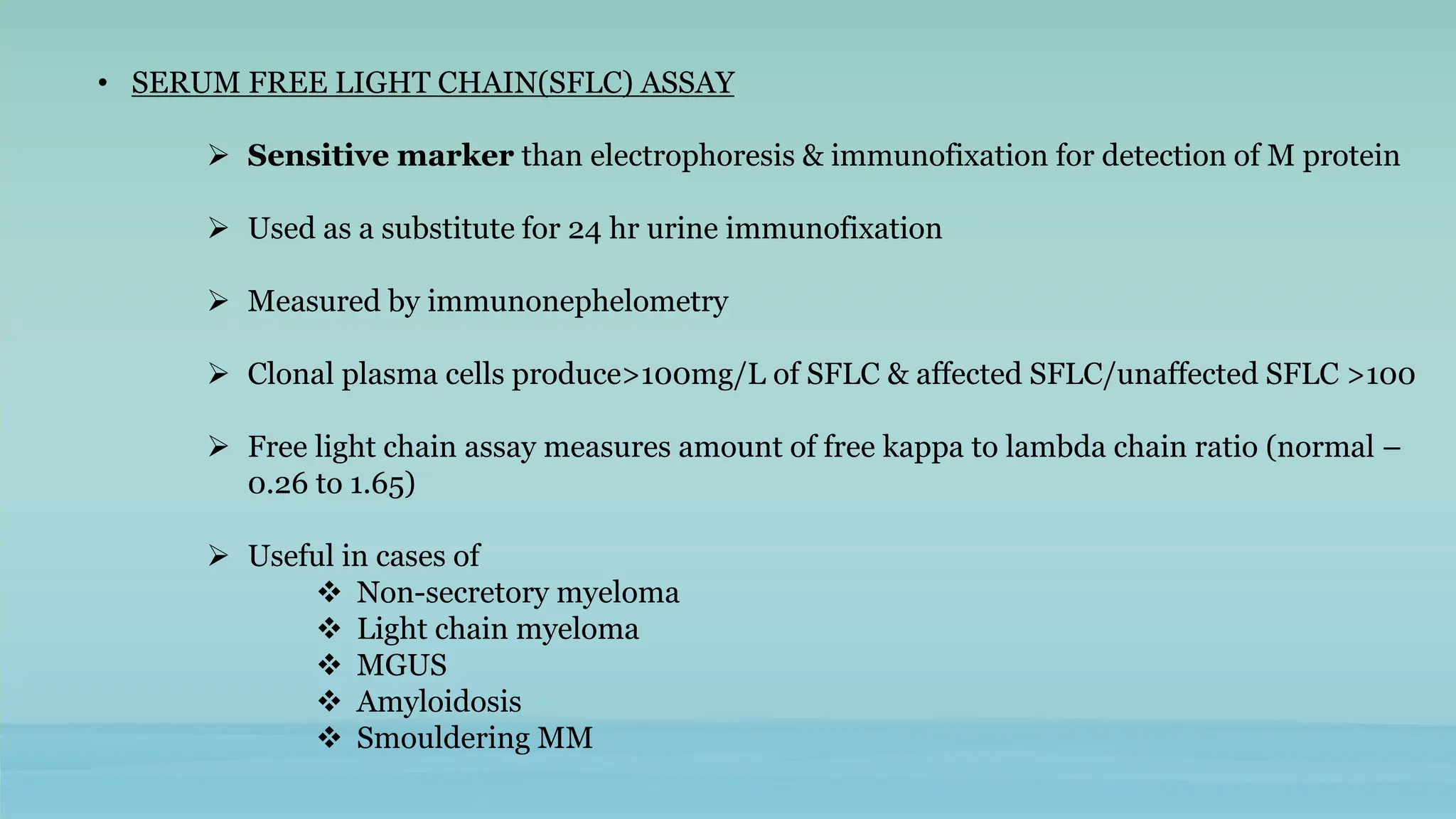
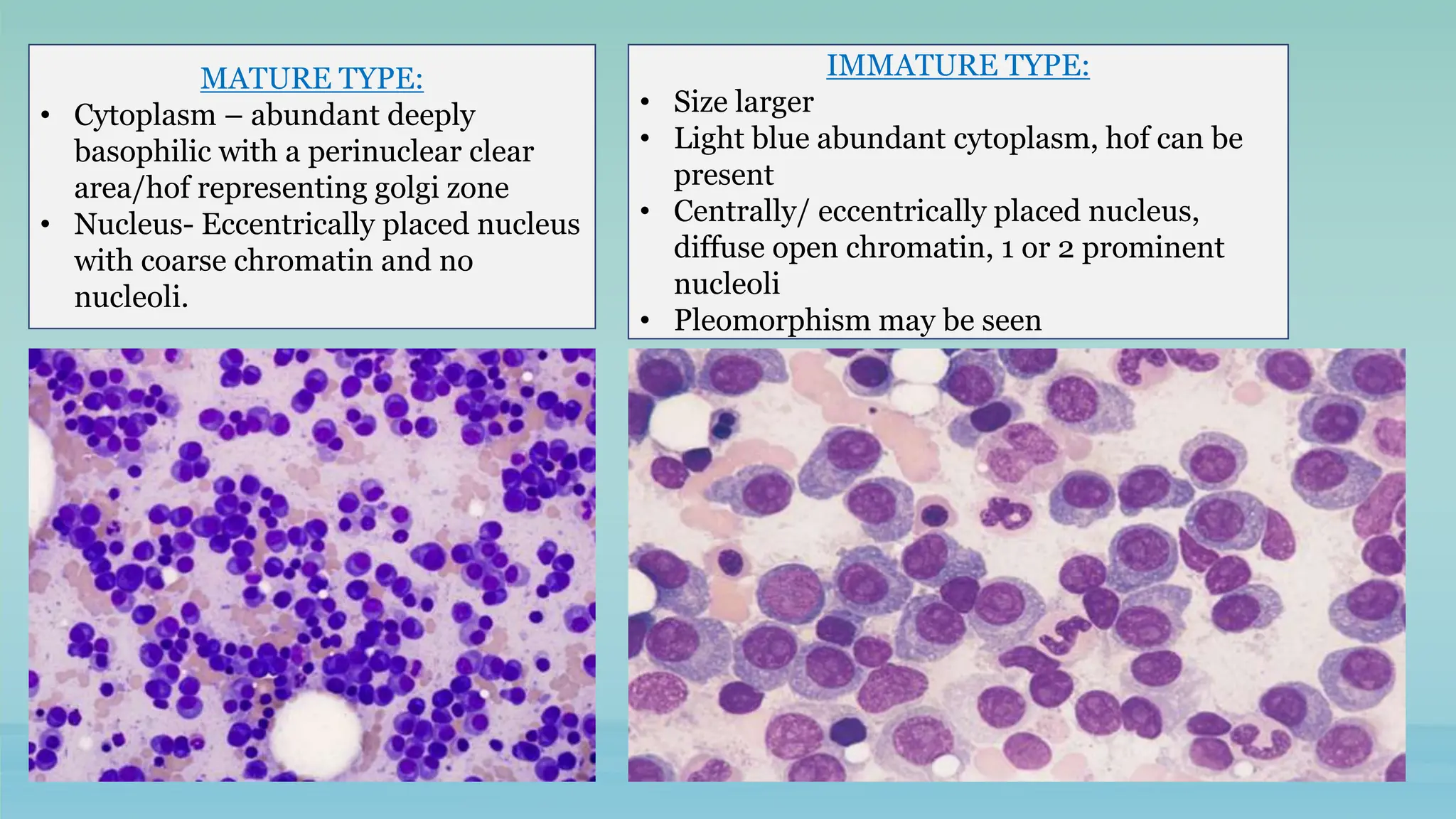
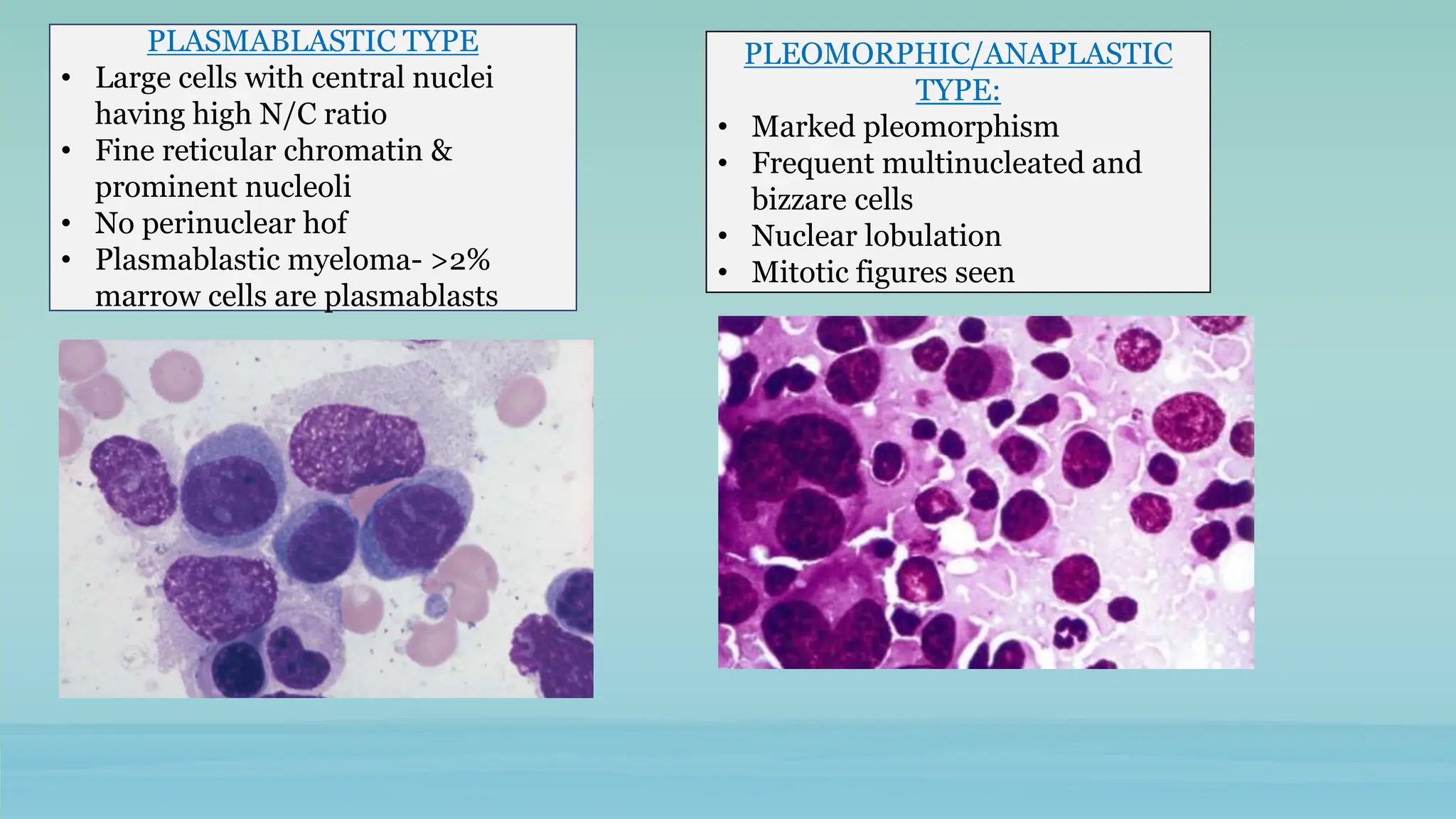
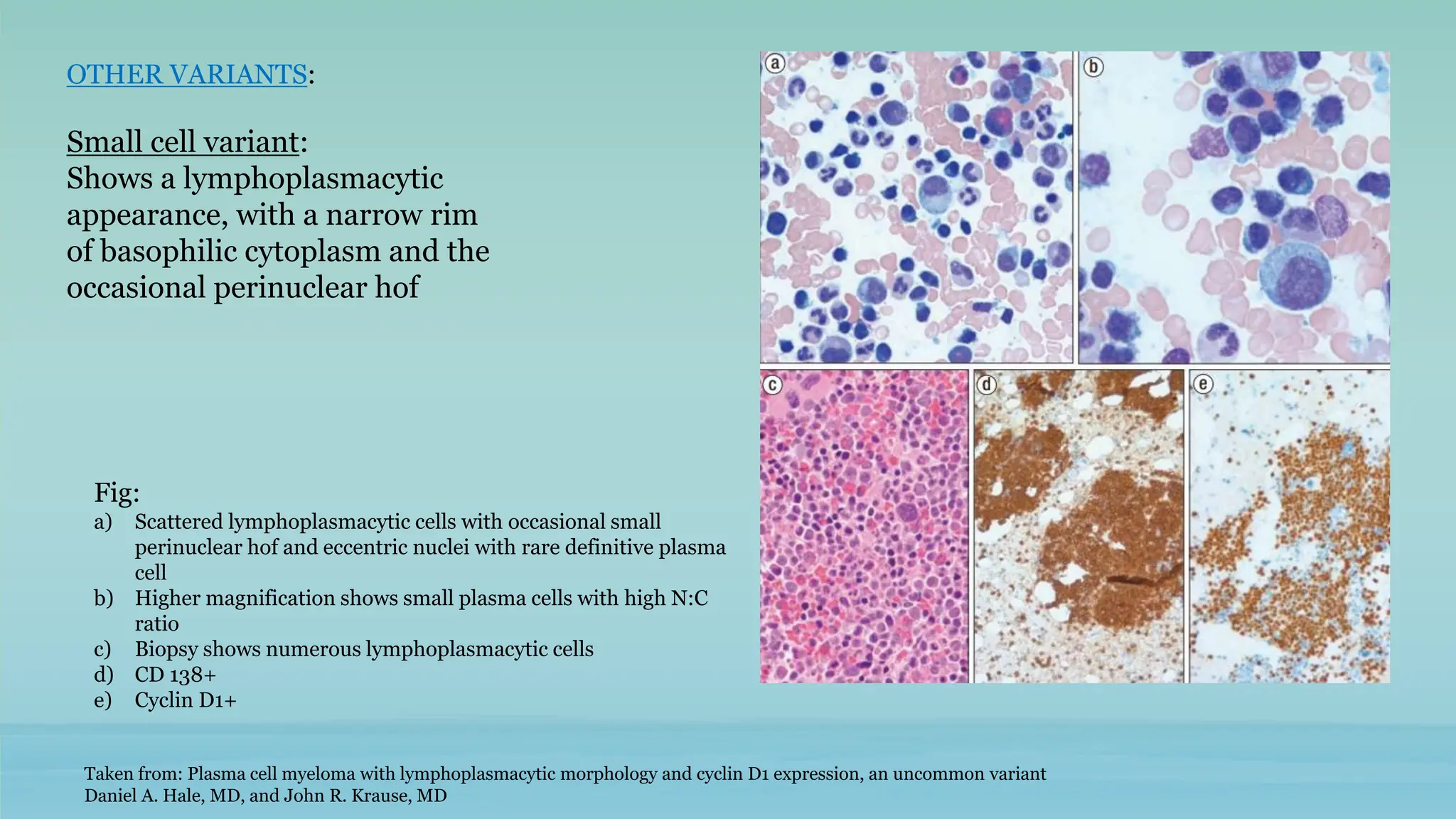
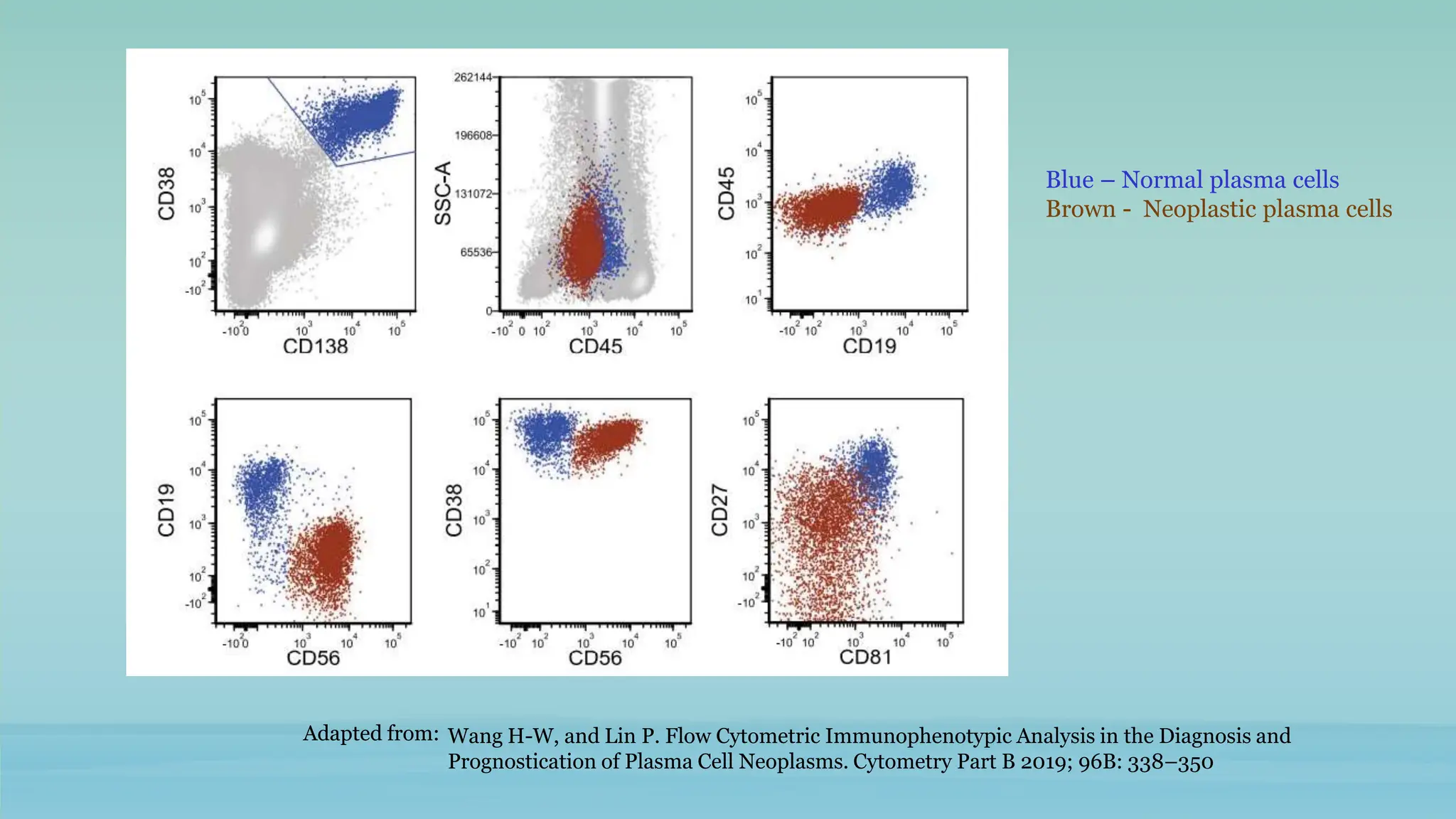
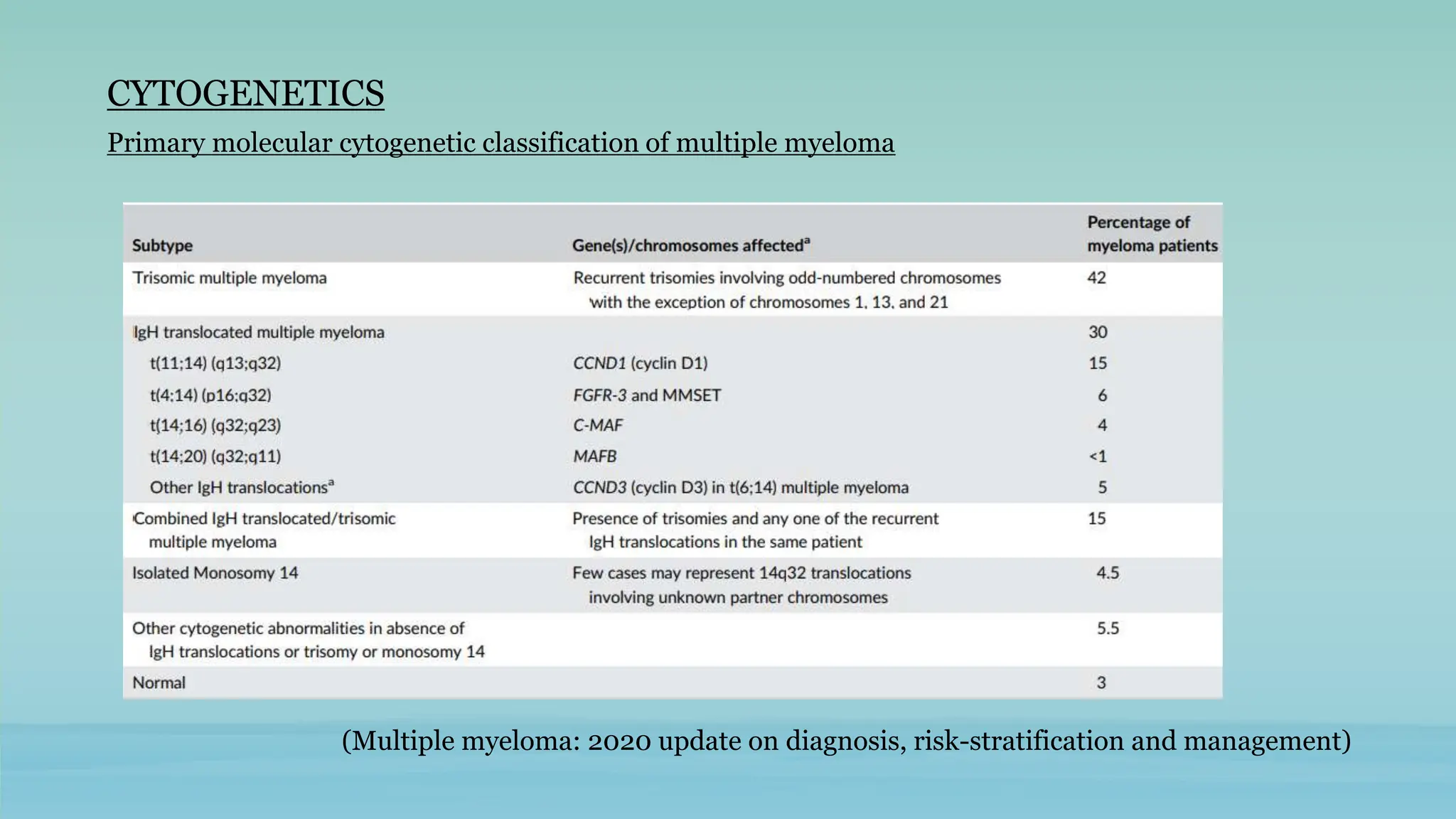
The document discusses plasma cell dyscrasias, characterized by the proliferation of a single clone of plasma cells producing monoclonal serum proteins. It covers various types such as MGUS, multiple myeloma, and related disorders, detailing their definitions, diagnostic criteria, clinical features, laboratory findings, and treatment options. Additionally, it explores the pathogenesis of multiple myeloma and techniques for diagnosis and monitoring, including flow cytometry and immunohistochemistry.