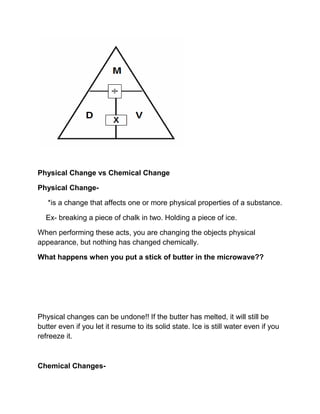
The document discusses physical and chemical properties of matter. It defines density as the mass per unit volume and provides the equation for calculating density. It distinguishes between physical and chemical changes, noting that physical changes do not alter the chemical makeup of a substance while chemical changes produce new substances.