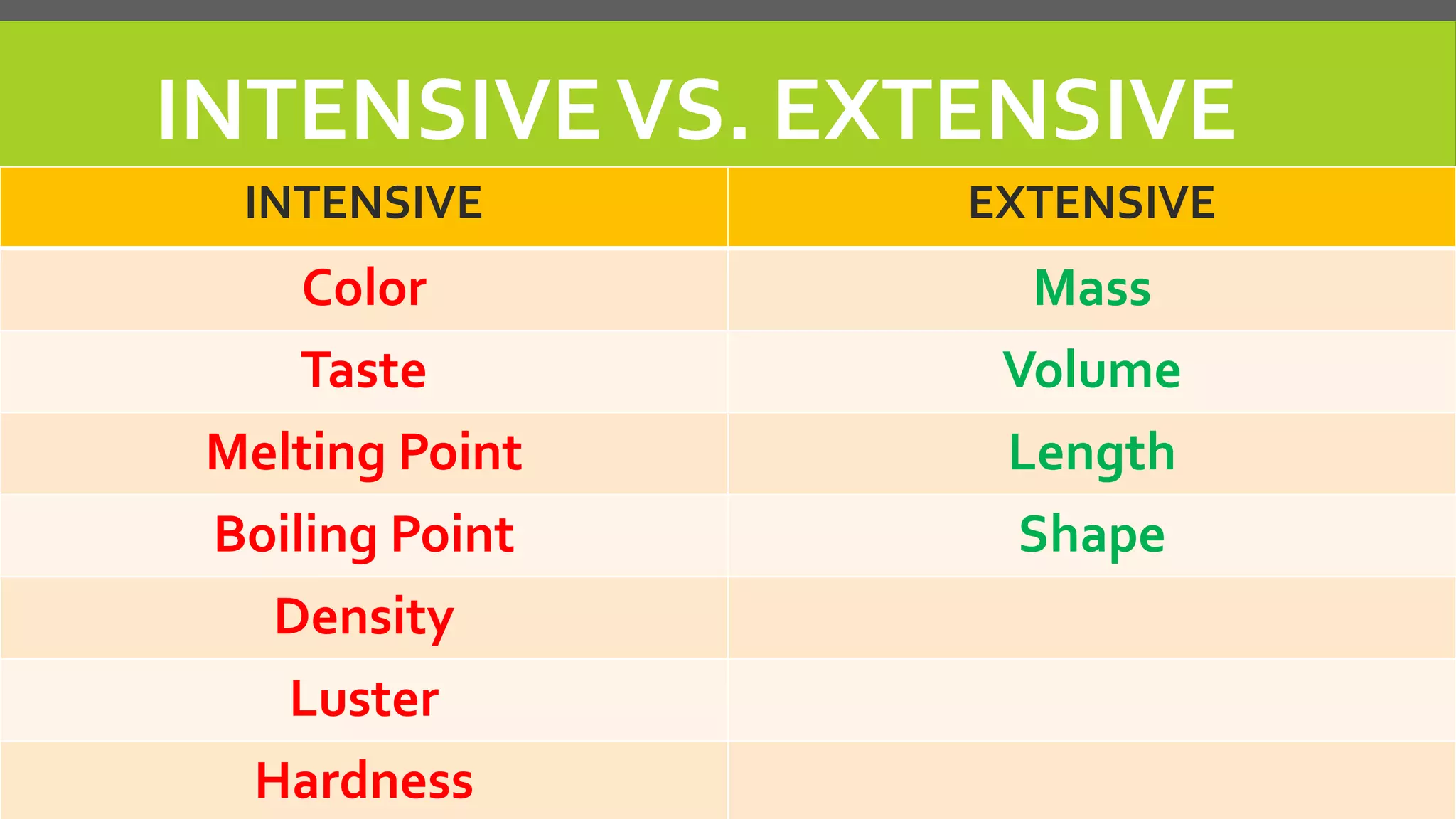
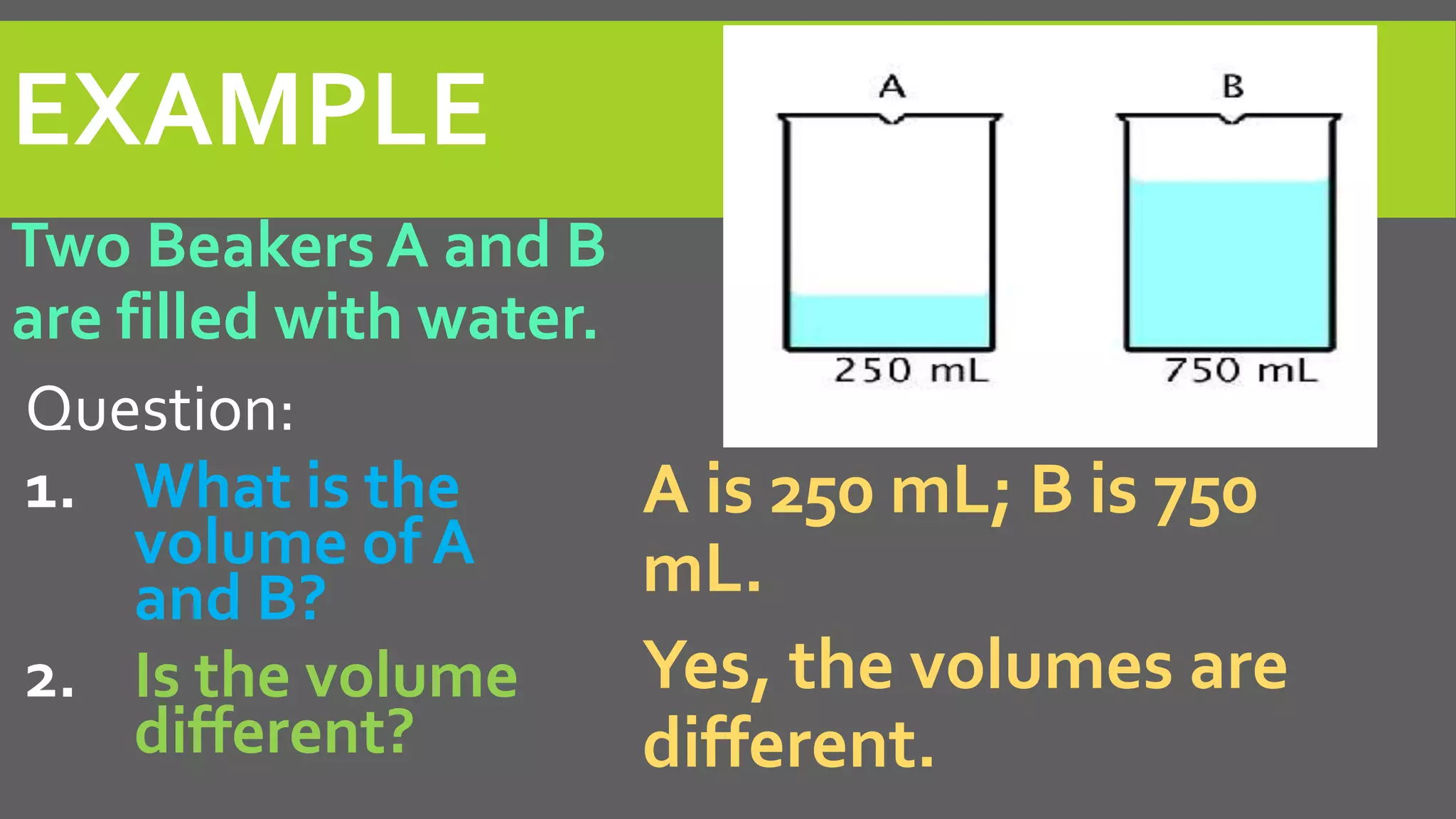
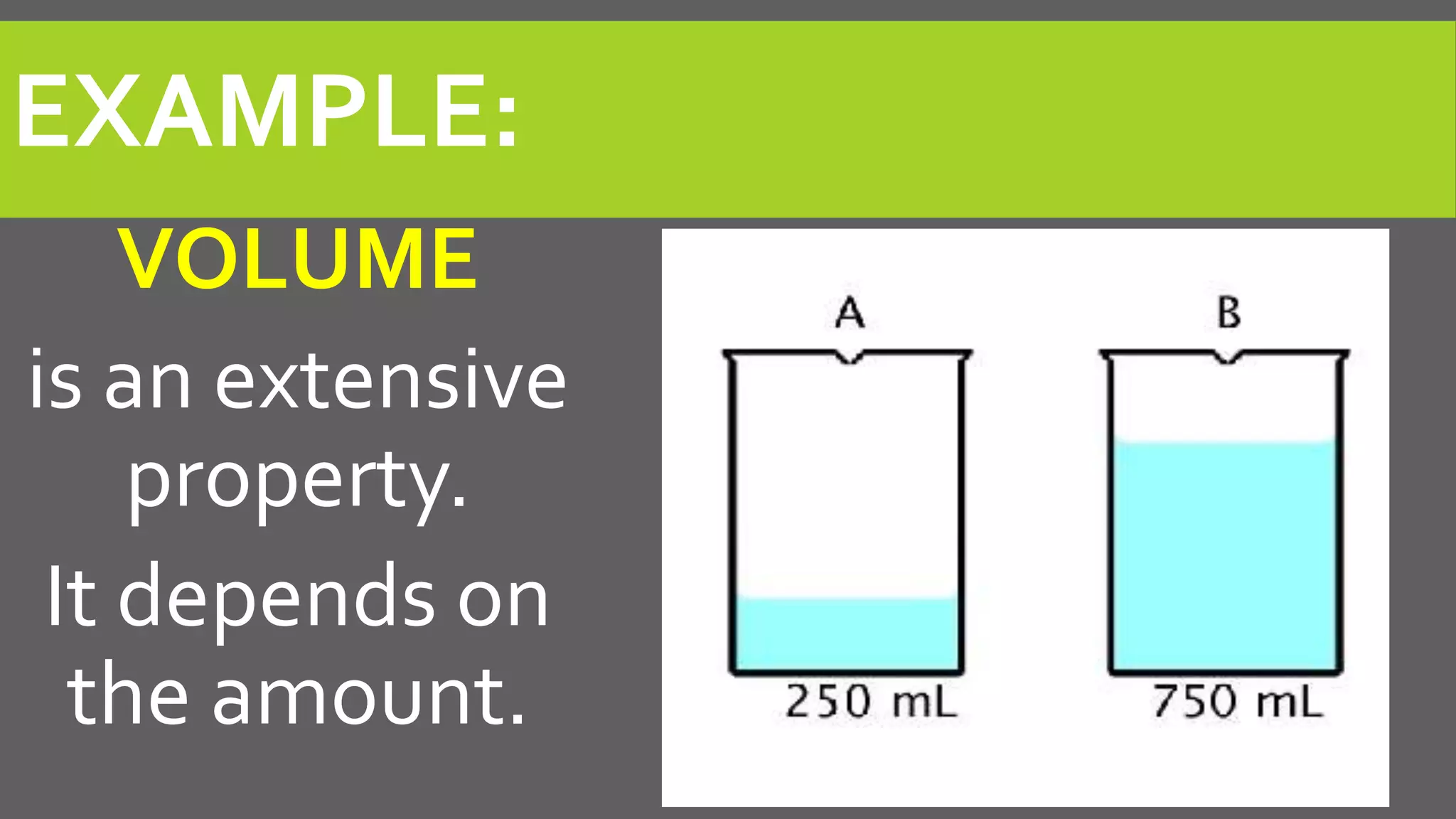
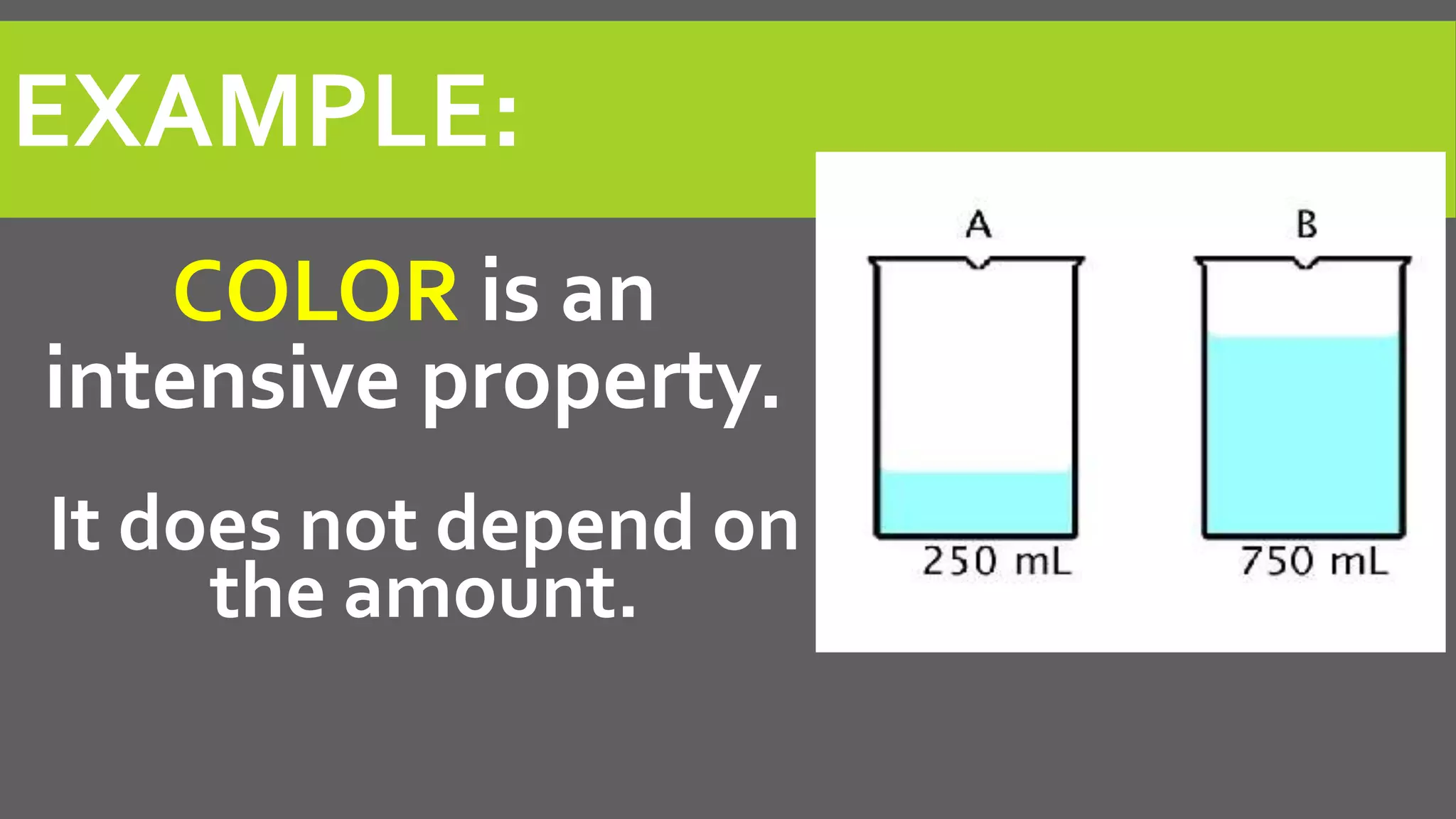
This document discusses properties of matter. It defines matter as anything that occupies space and has mass. It describes two types of properties: physical properties and chemical properties. Physical properties do not change the composition of matter and include intensive properties like density that do not depend on amount, and extensive properties like volume that do depend on amount. Chemical properties involve a change in composition or formula through chemical reactions. Examples of intensive and extensive properties and physical and chemical changes are provided. The document concludes with an assignment to answer questions in a notebook by a certain date.