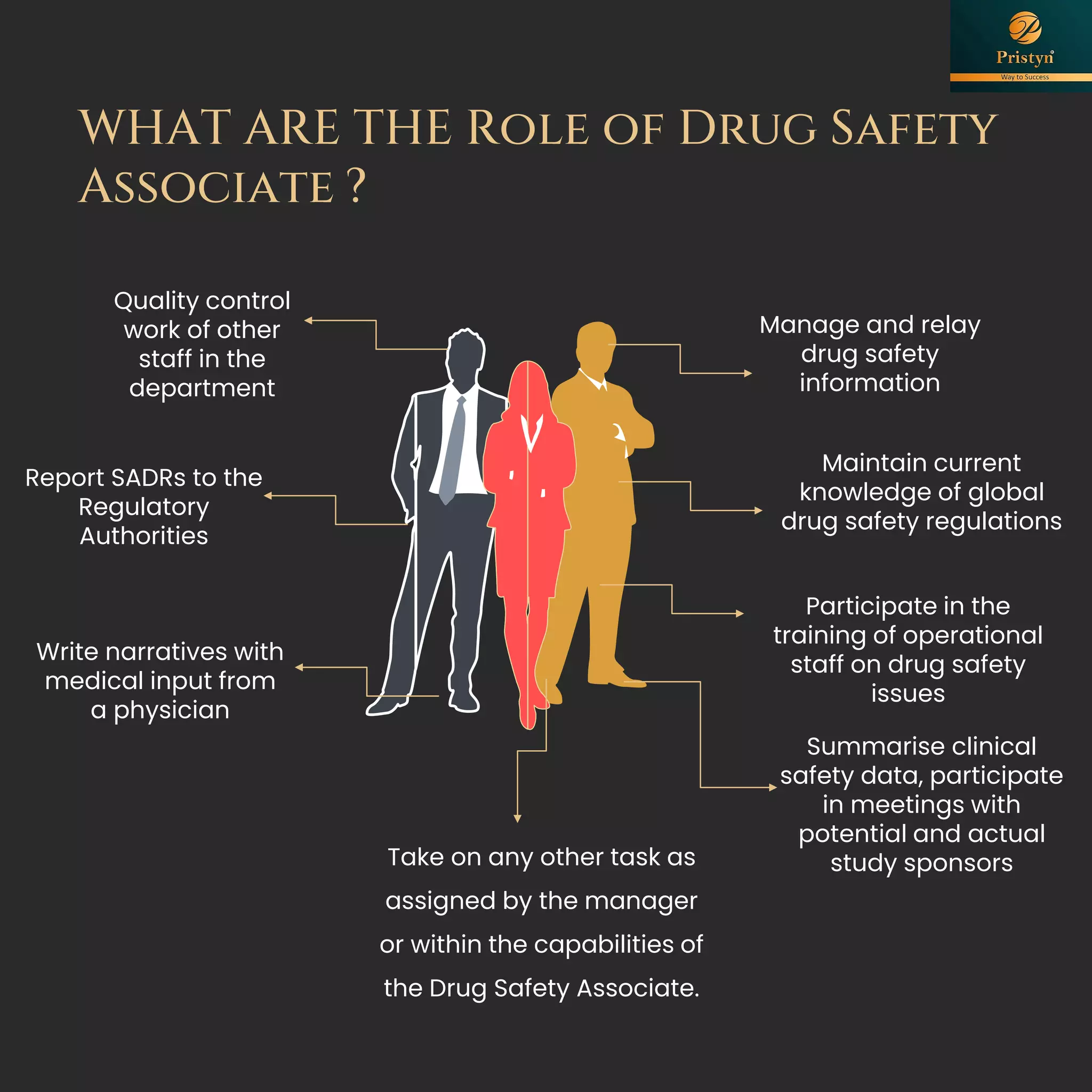
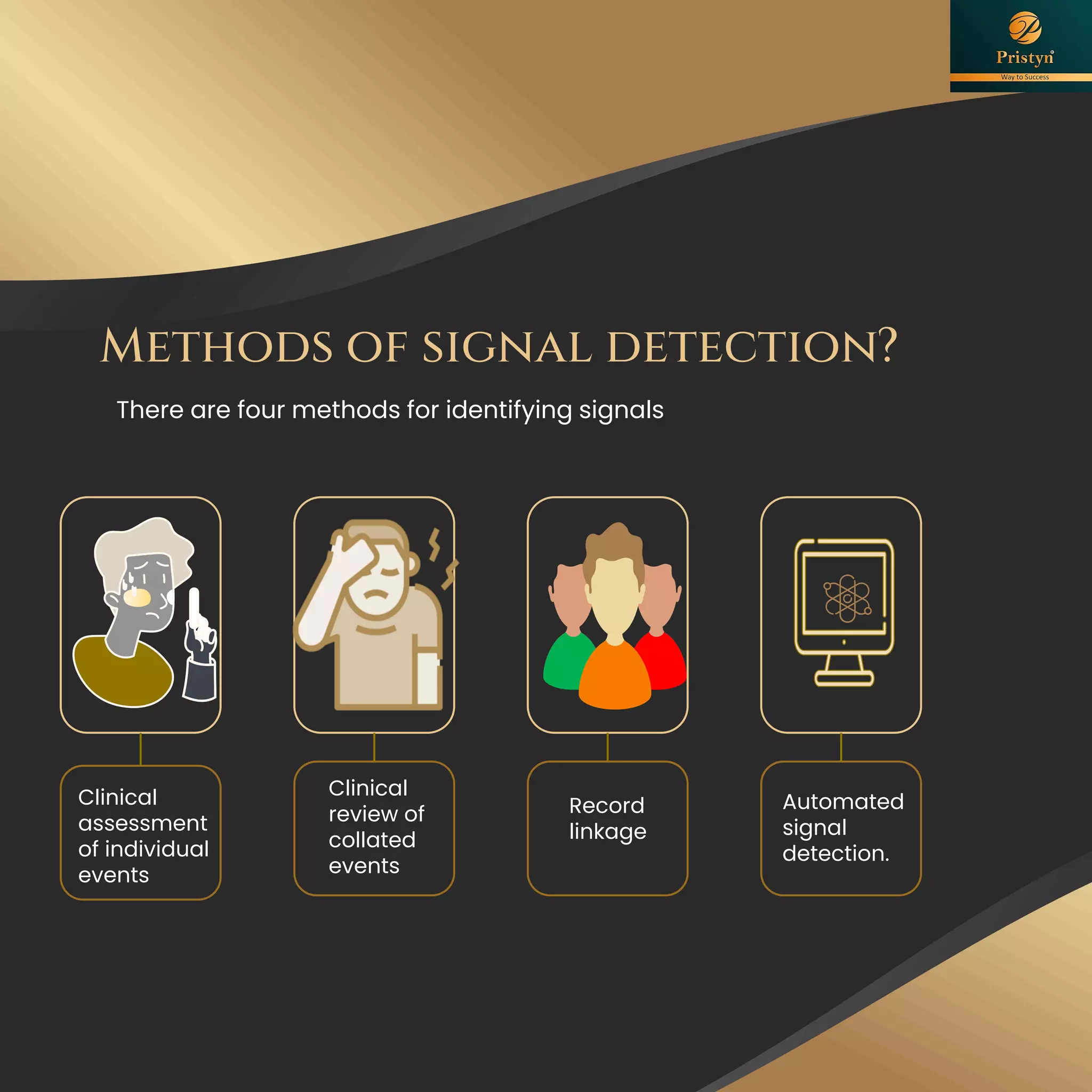
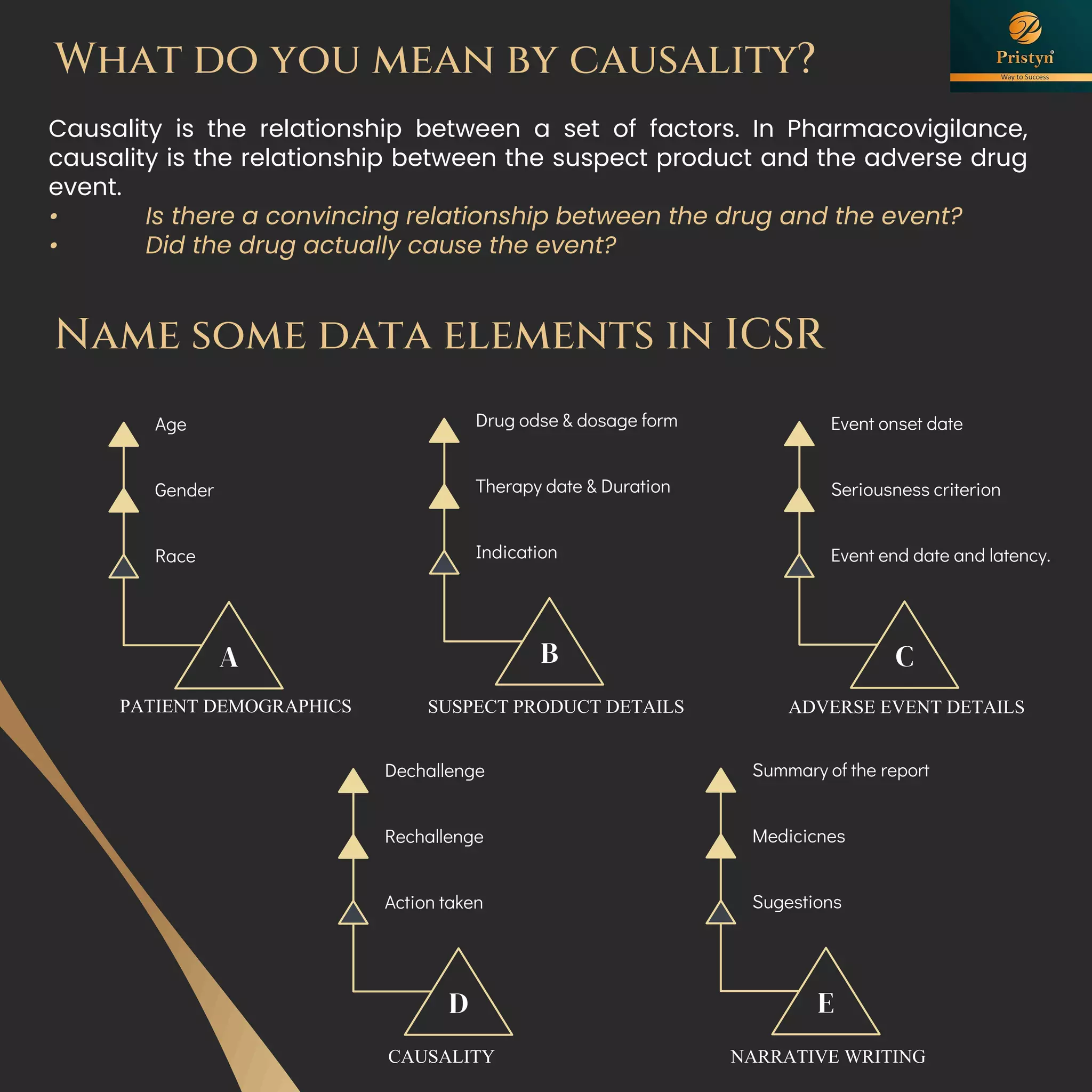
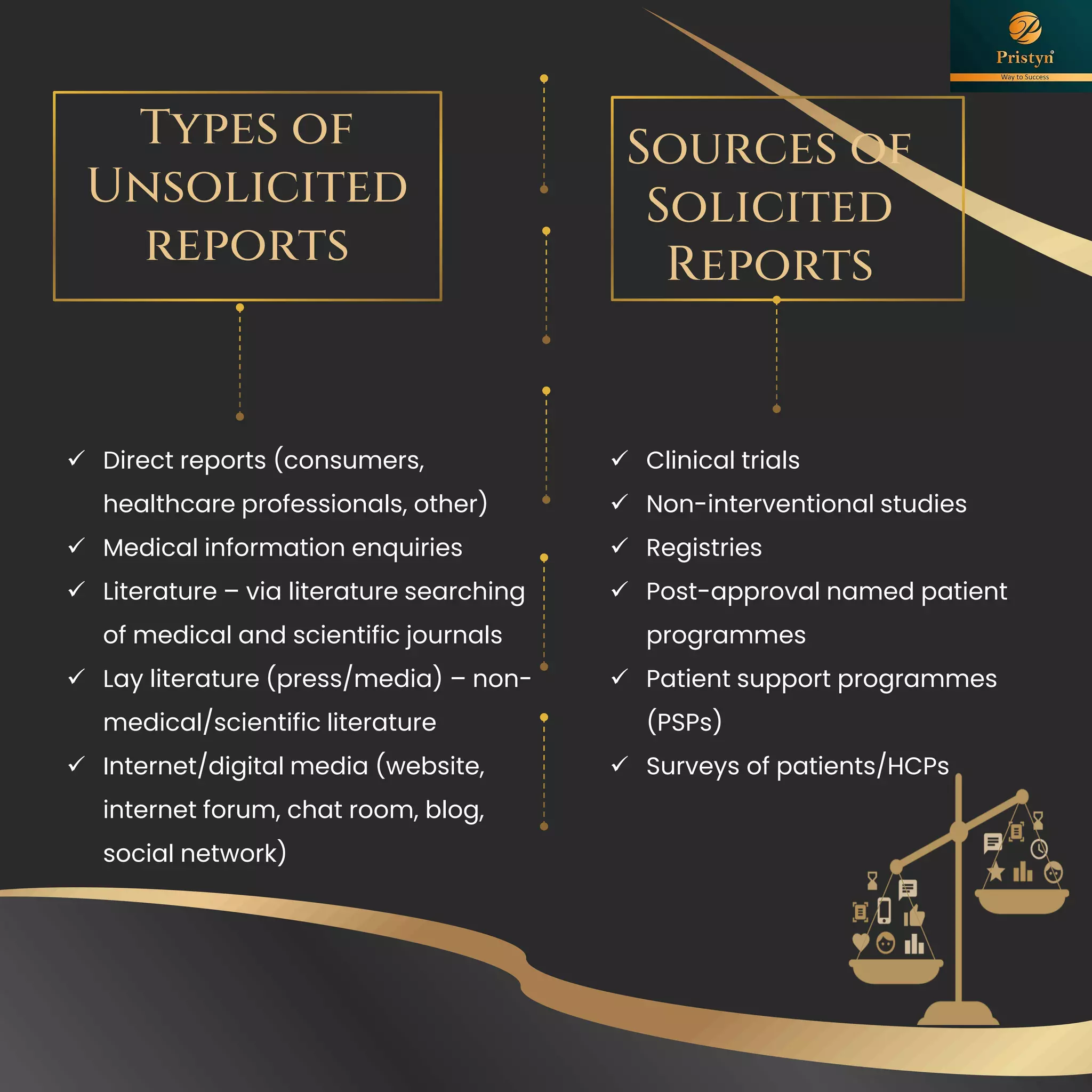
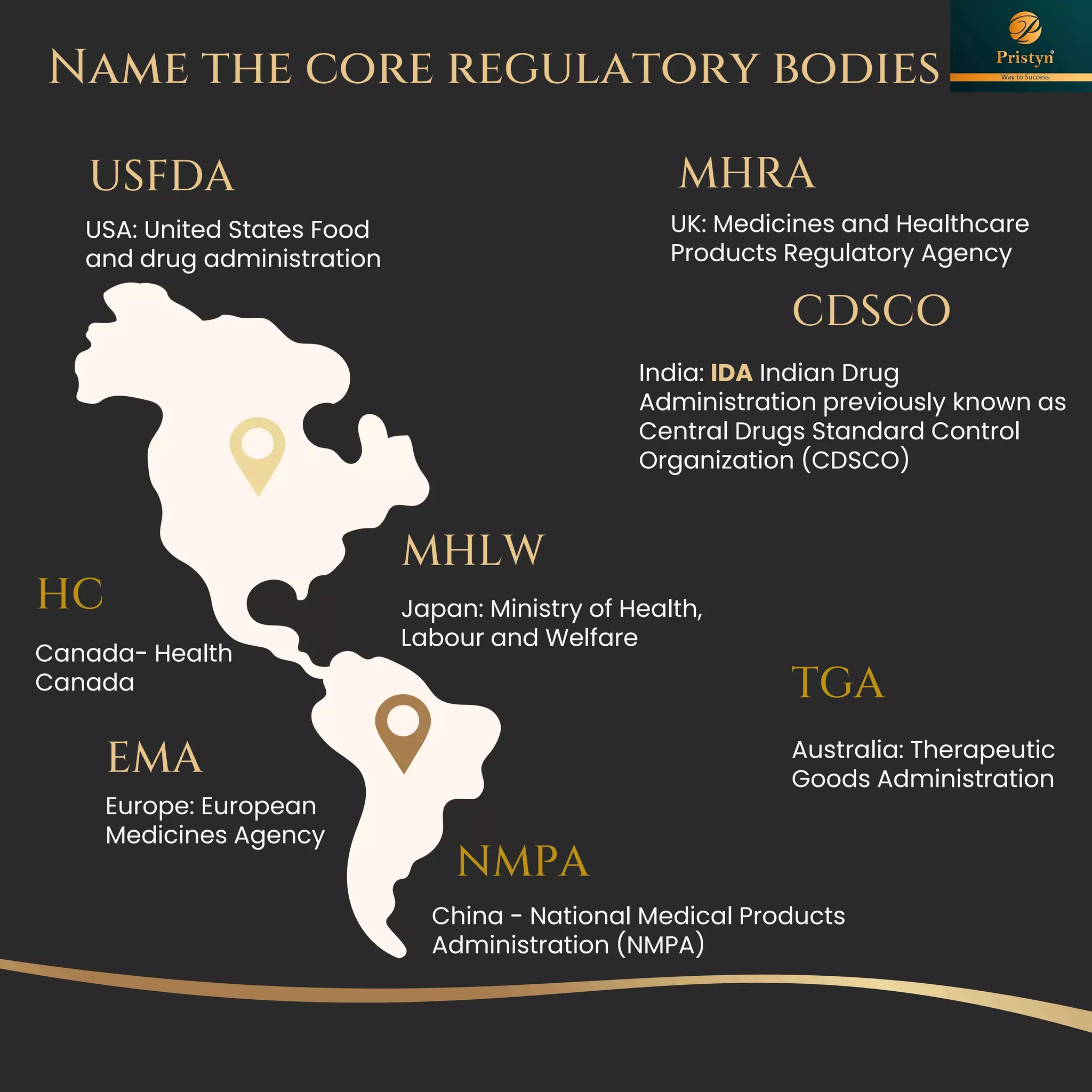
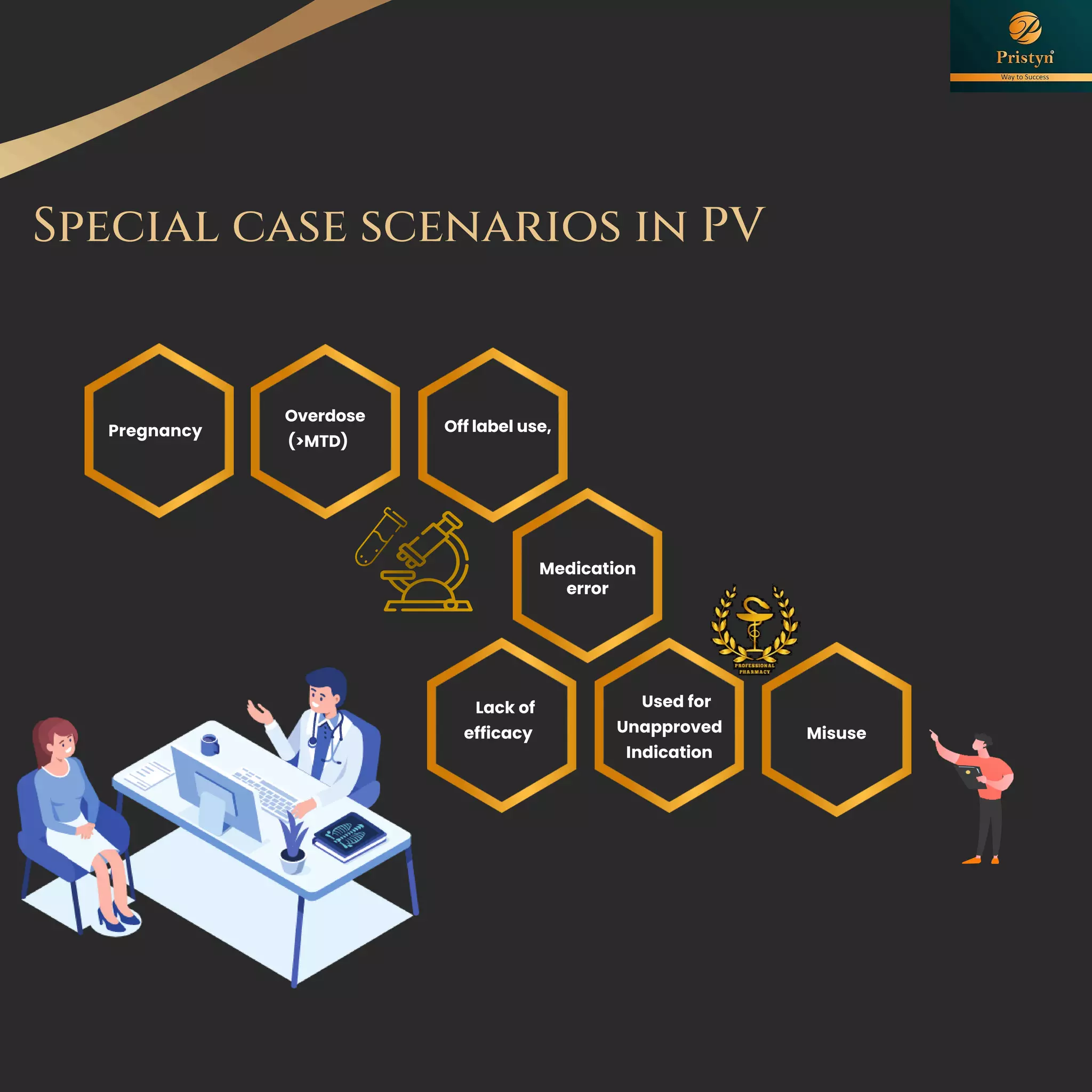
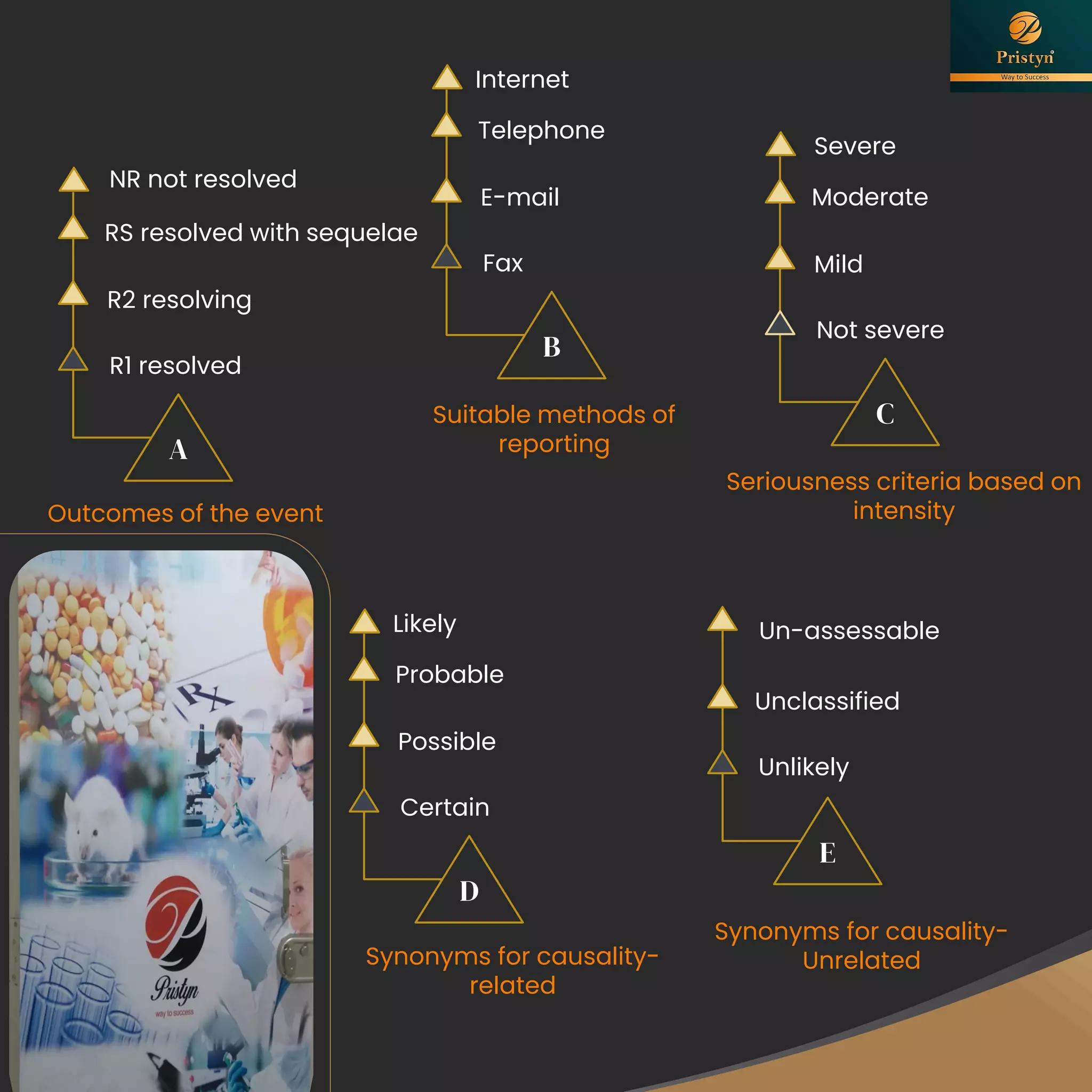
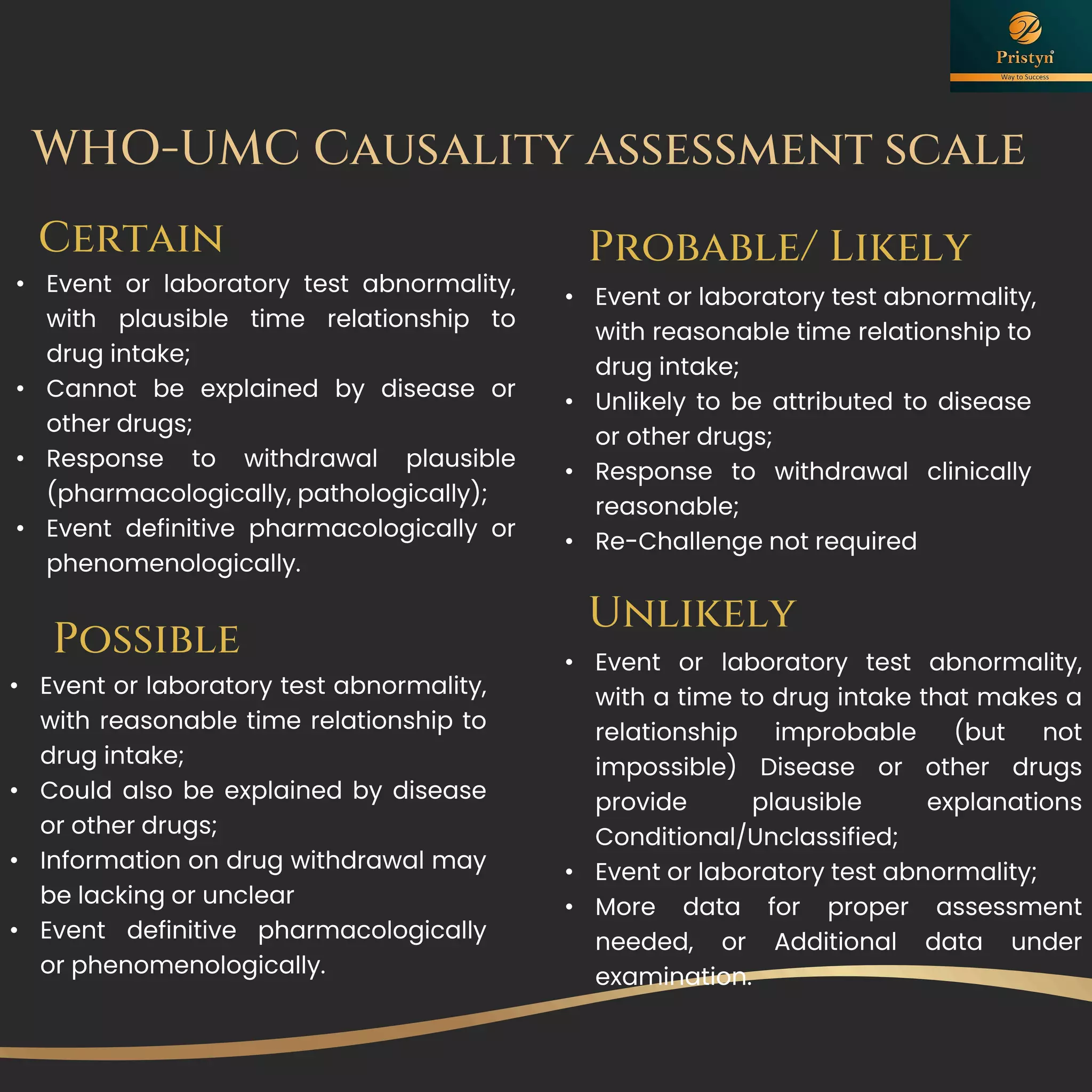
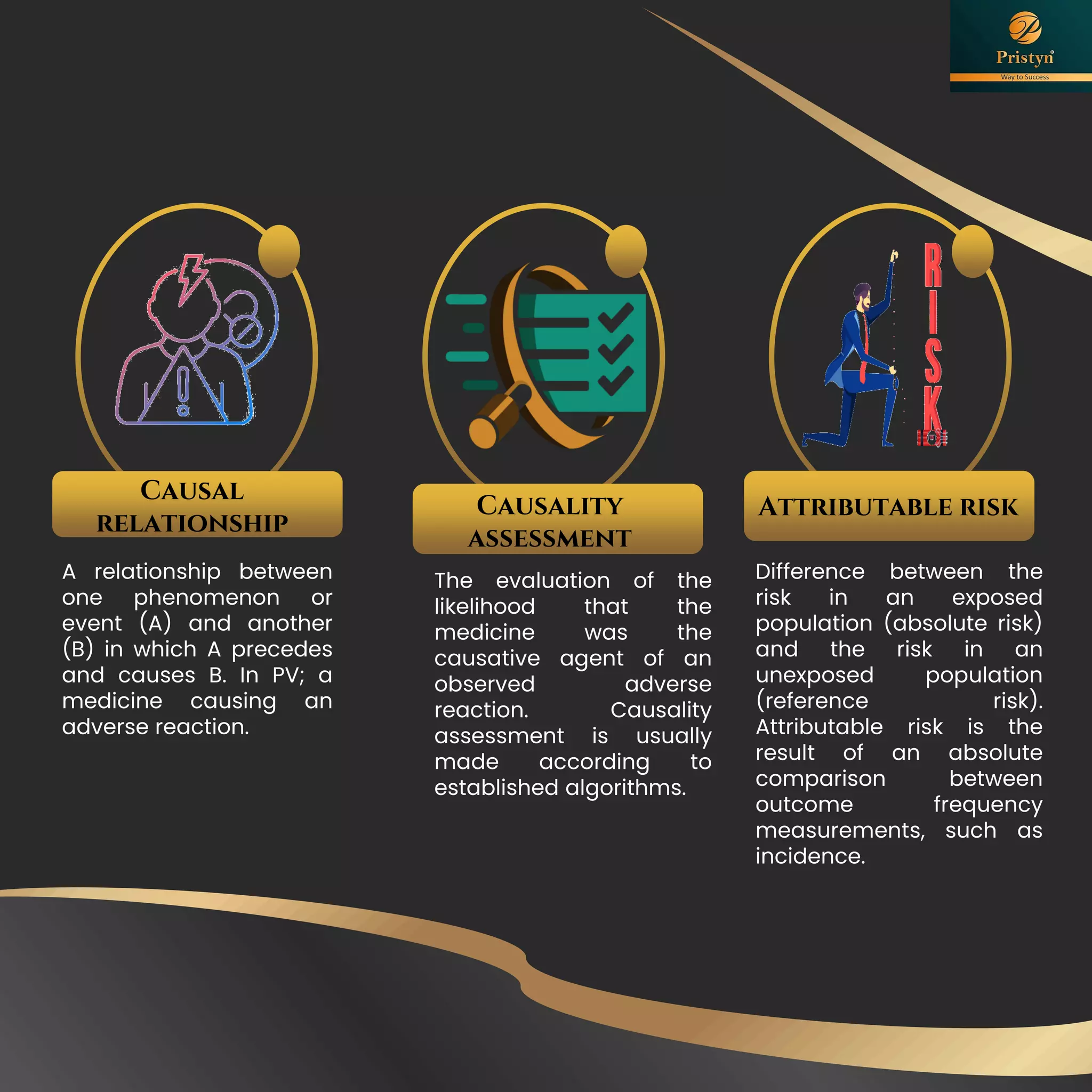
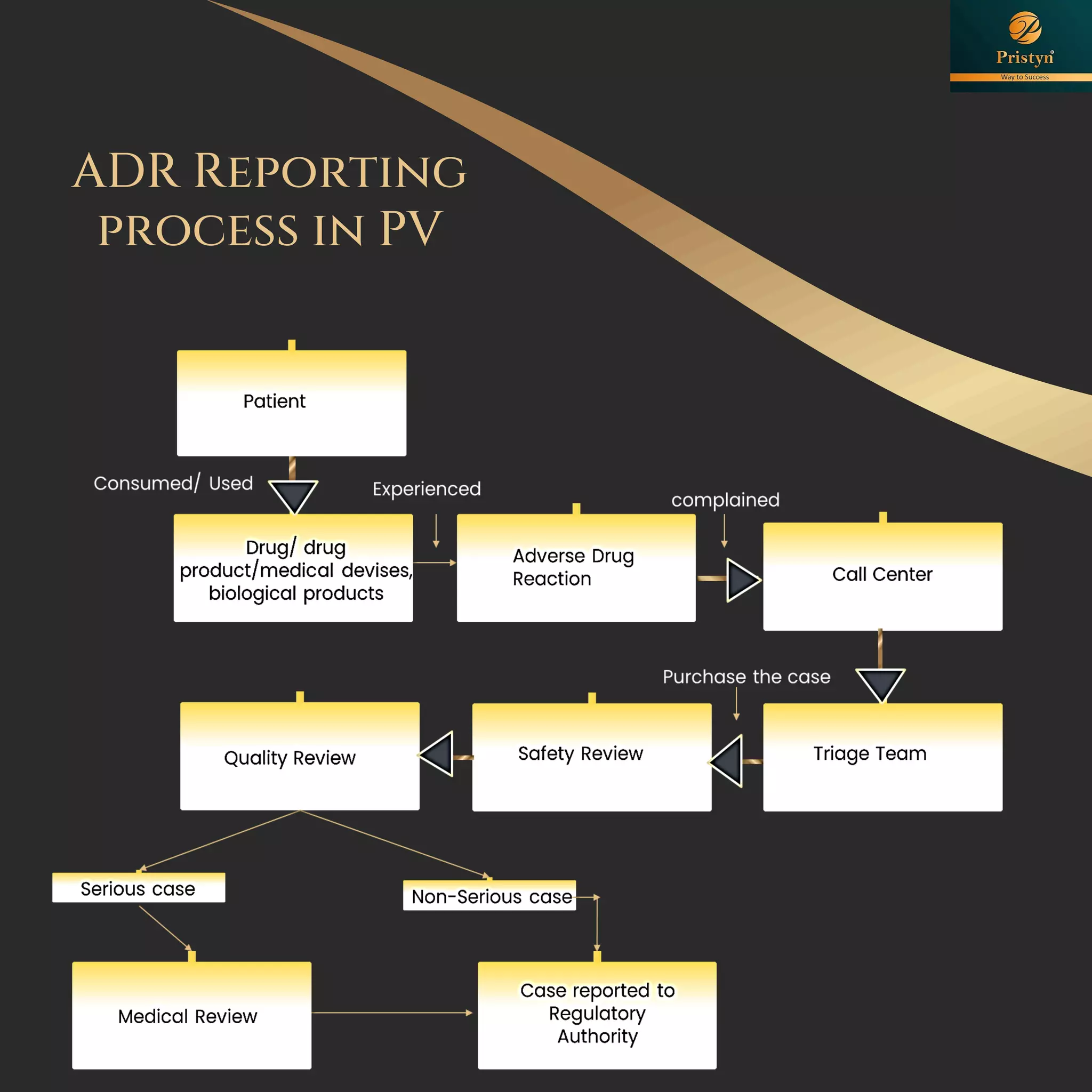
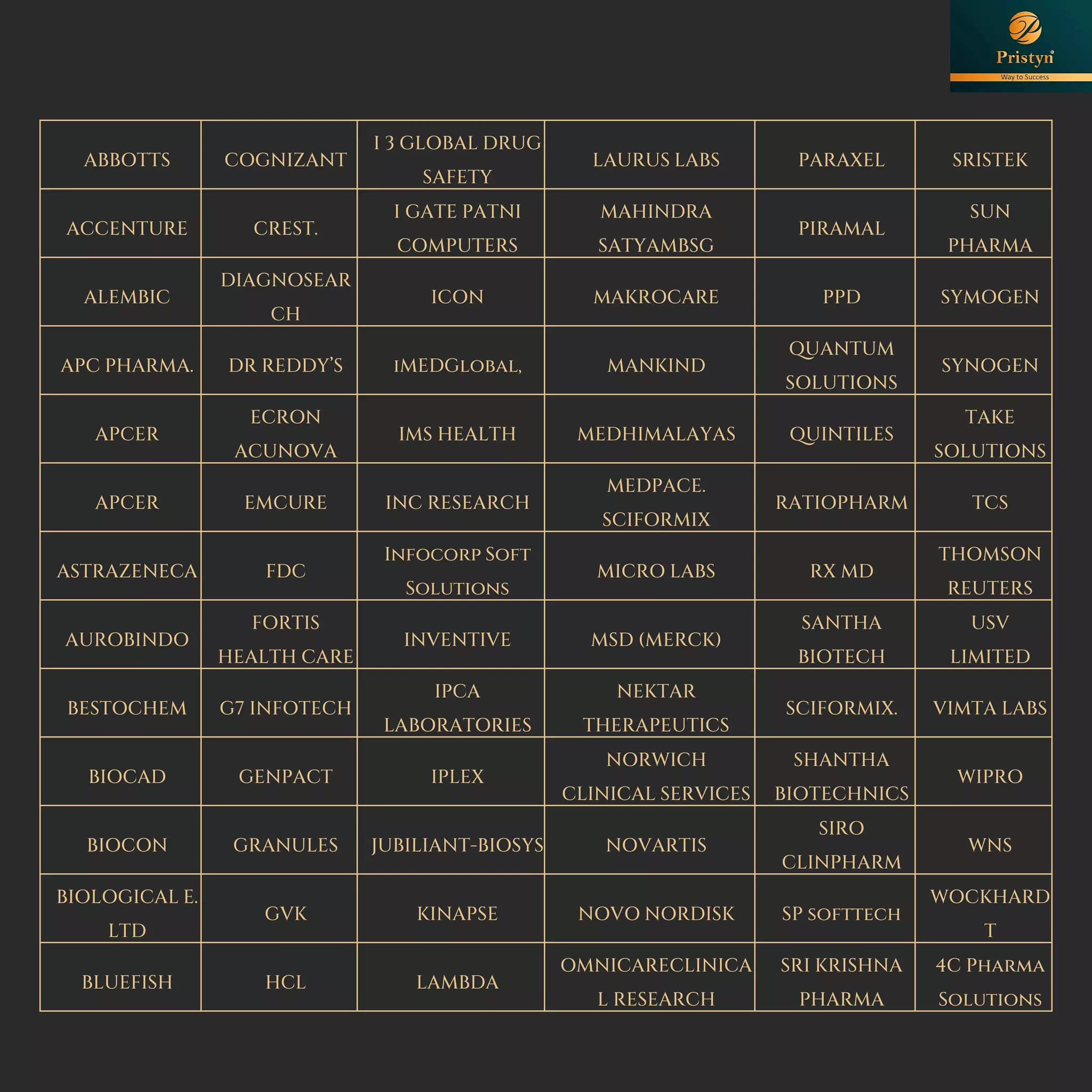
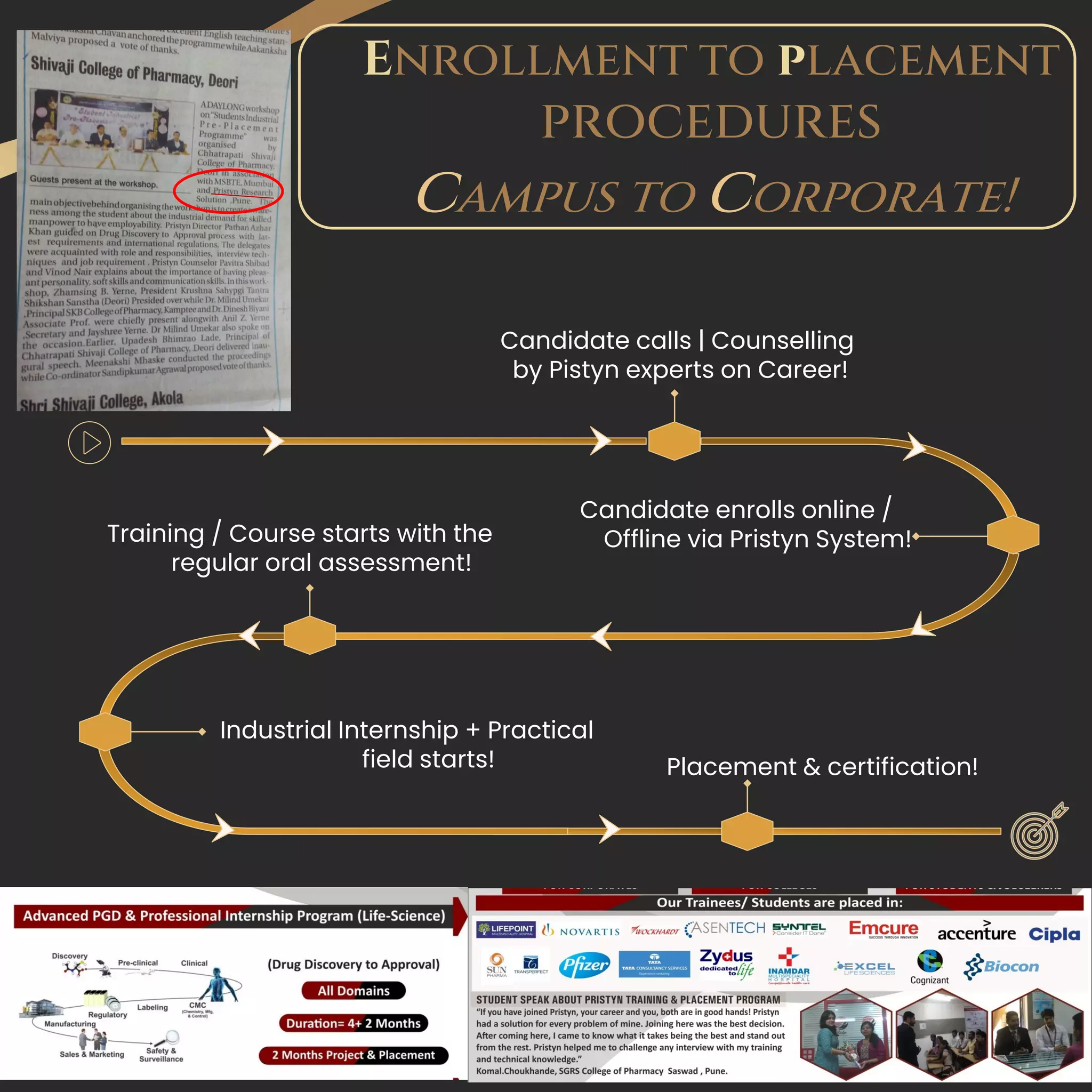
The document serves as a comprehensive guide for job seekers in pharmacovigilance, outlining key concepts, definitions, objectives, and roles within the field. It also discusses important regulations, methodologies for signal detection, reporting timelines for adverse events, and the significance of pharmacovigilance in drug safety and efficacy. Additionally, it highlights essential resources and organizations relevant to pharmacovigilance practices in India and globally.