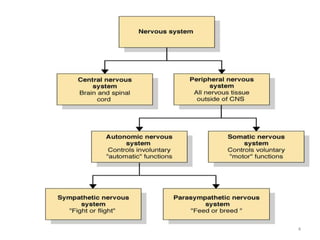
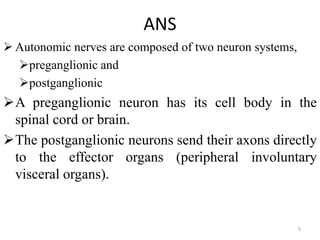
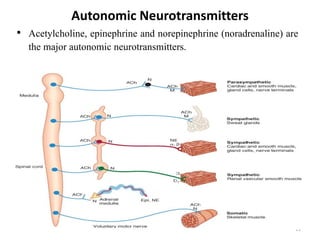
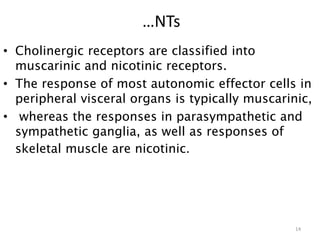
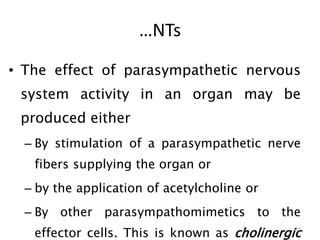
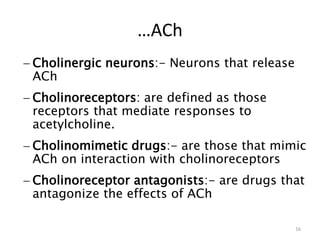
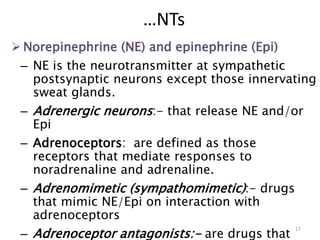
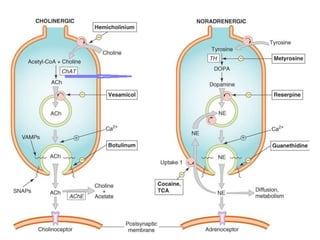
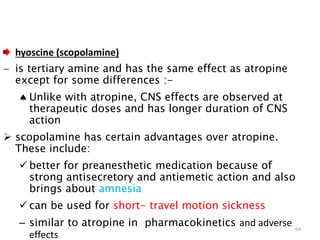
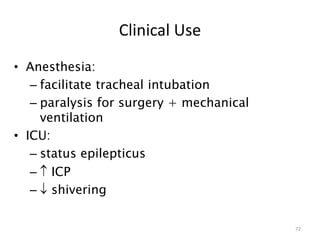
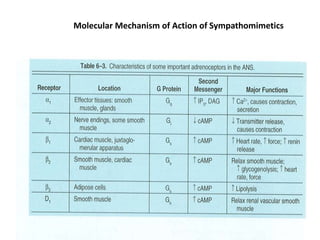
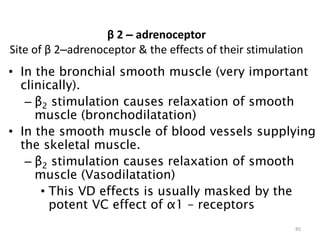
This document provides an overview of the autonomic nervous system (ANS) and autonomic drugs. It begins by outlining the objectives and reviewing the physiology of the ANS, distinguishing the sympathetic and parasympathetic nervous systems. It then discusses the major neurotransmitters of the ANS (acetylcholine, epinephrine, norepinephrine) and how different classes of autonomic drugs (sympathomimetics, parasympathomimetics) act on the sympathetic and parasympathetic systems. Specific examples are provided of cholinergic drugs and their clinical uses and effects in various organ systems.