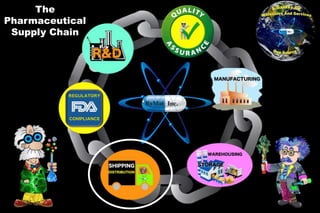
The document discusses the complexities and challenges of customs clearance in the pharmaceutical supply chain, highlighting issues such as poor planning, bureaucratic processes, and political changes that can impact delivery times and costs. Strategies for improvement include leveraging preshipment inspections, building capacity within consignees, consolidating shipments, and establishing metrics for performance evaluation. It emphasizes the importance of selecting reliable clearing agents and considering alternative ports to enhance efficiency in the supply chain.