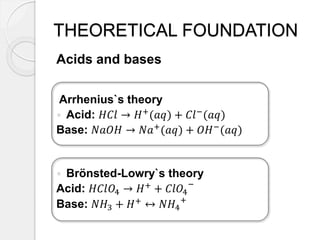
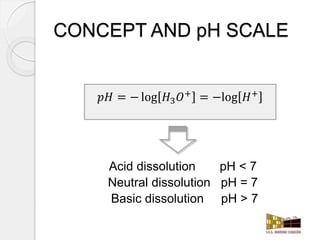
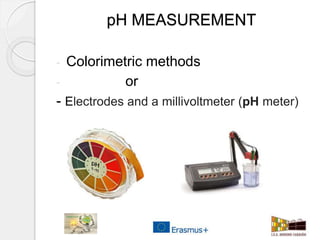
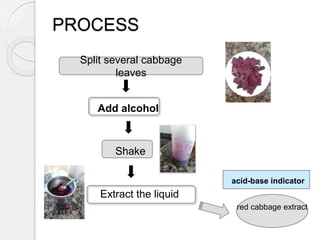
This document outlines how to make a homemade pH indicator from red cabbage and use it to measure the pH of common household substances. It discusses acid-base theories, defines pH as the negative log of the hydrogen ion concentration, and explains that pH values below 7 indicate acids and above 7 indicate bases. The materials and process for preparing the red cabbage extract and using it with a pH scale to test the acidity of substances like lemons, oranges, bleach, water, toothpaste, vinegar, and milk are described.