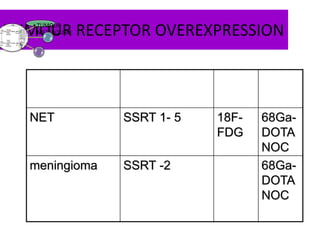
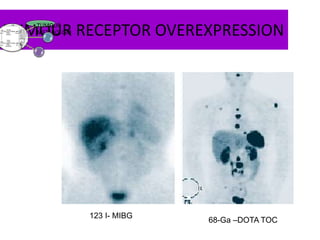
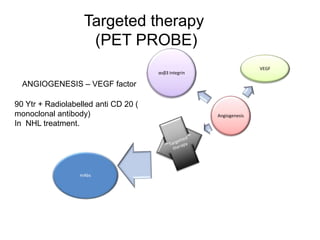
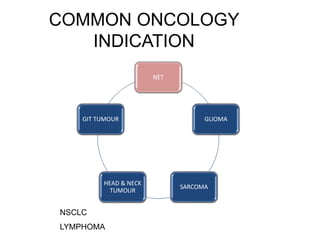
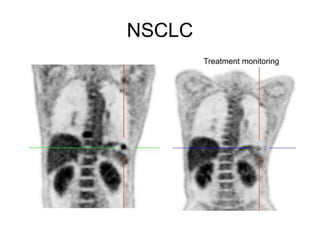
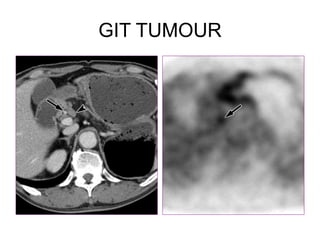
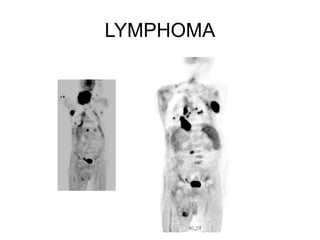
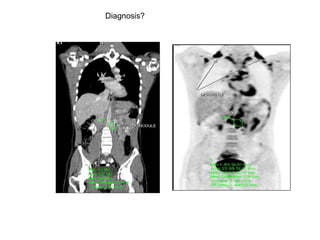
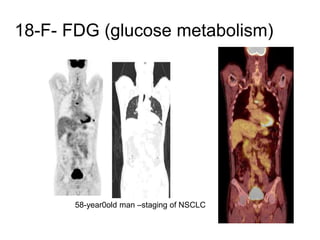
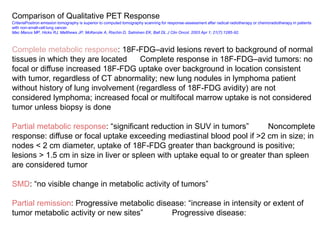
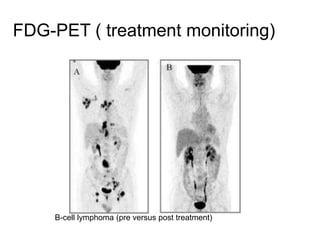
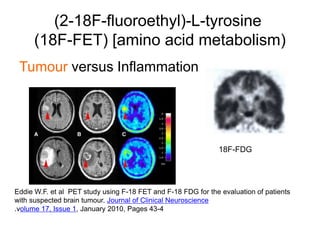
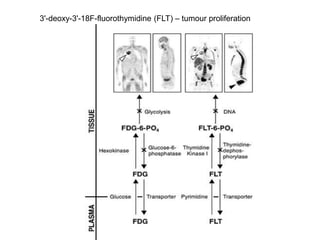
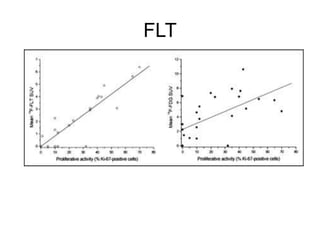
The document discusses the synthesis and application of PET tracers in oncology, highlighting their role in imaging tumor metabolism, receptor overexpression, and hypoxia. It outlines criteria for assessing treatment response in diseases such as non-small-cell lung cancer and mentions various PET tracers like 18F-FDG and 18F-FET, along with their implications for treatment monitoring and prognosis. Additionally, it emphasizes the superiority of PET over CT for response assessment and the significance of standard uptake values (SUV) in predicting patient outcomes.


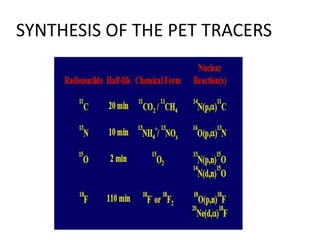
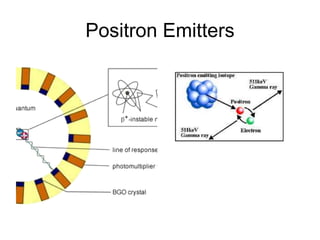
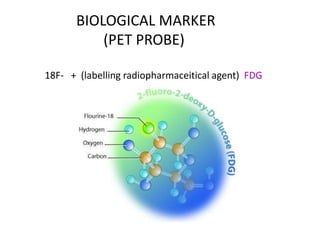
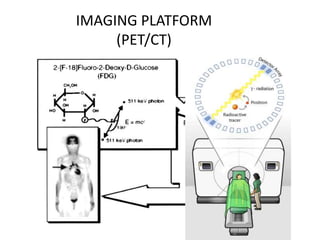

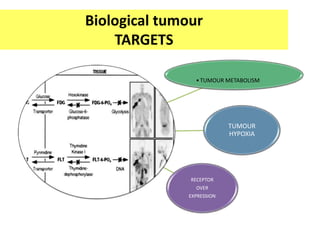

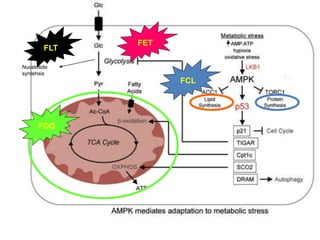



![TUMOUR HYPOXIA
•TUMOUR
METABOLISM
[18F]fluoroazomycin arabinoside (FAZA)
Feasibility of of 18F- FMISO PET imaging to measure hypoxia in patient
With glioblastoma
spenser et al.](https://image.slidesharecdn.com/petctonco1-191211143008/85/PET-CT-in-Oncology-24-320.jpg)