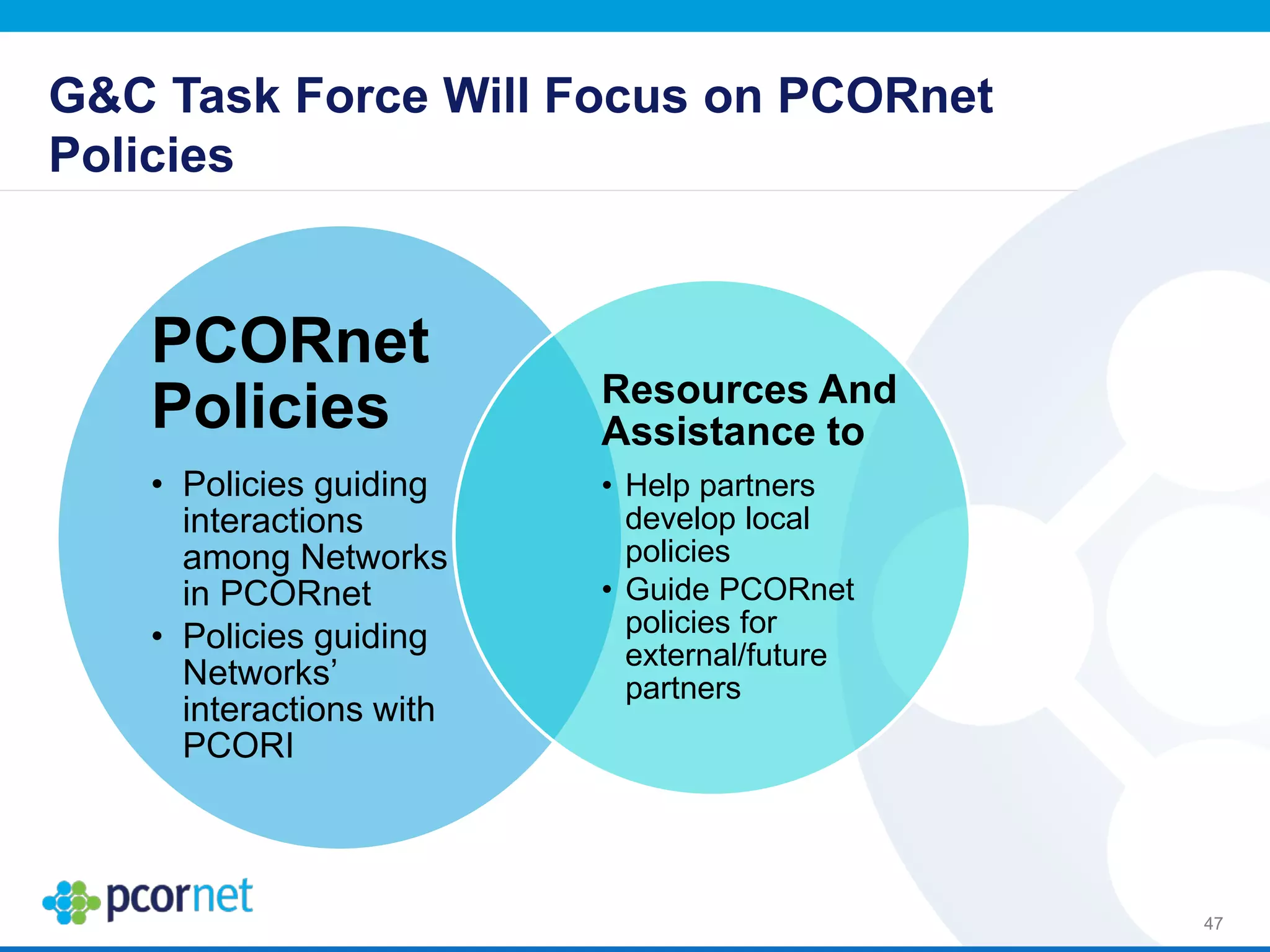
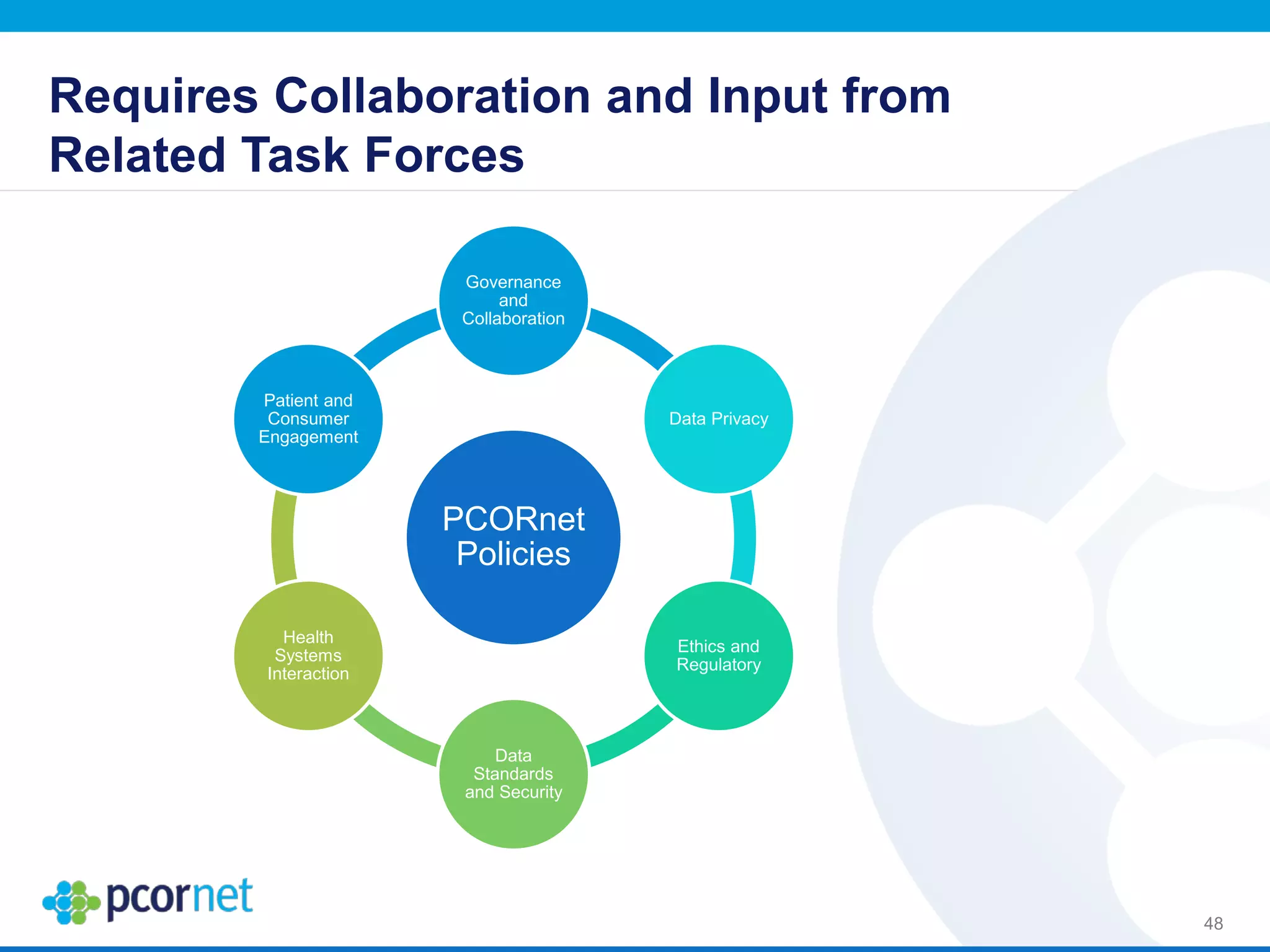
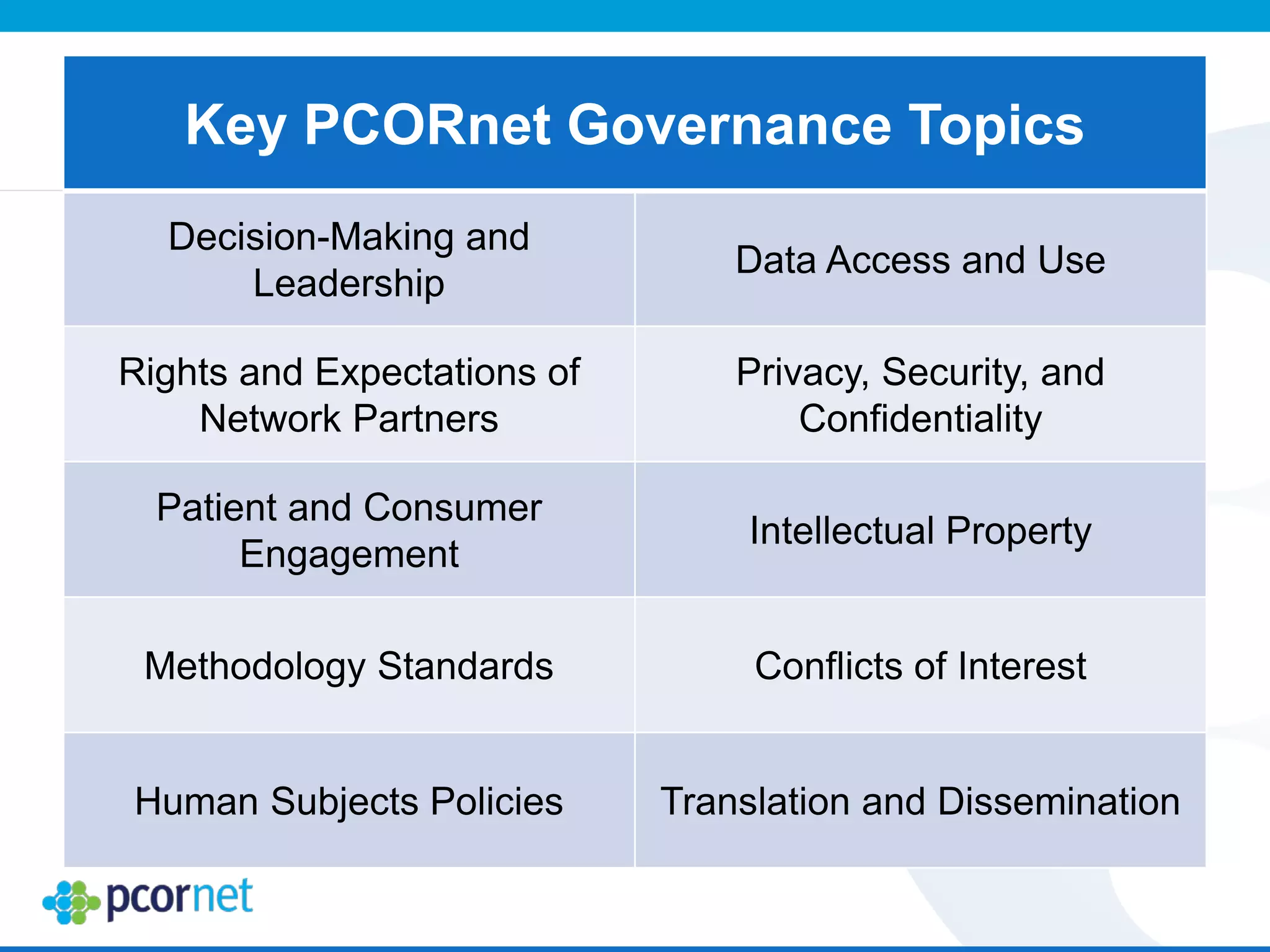
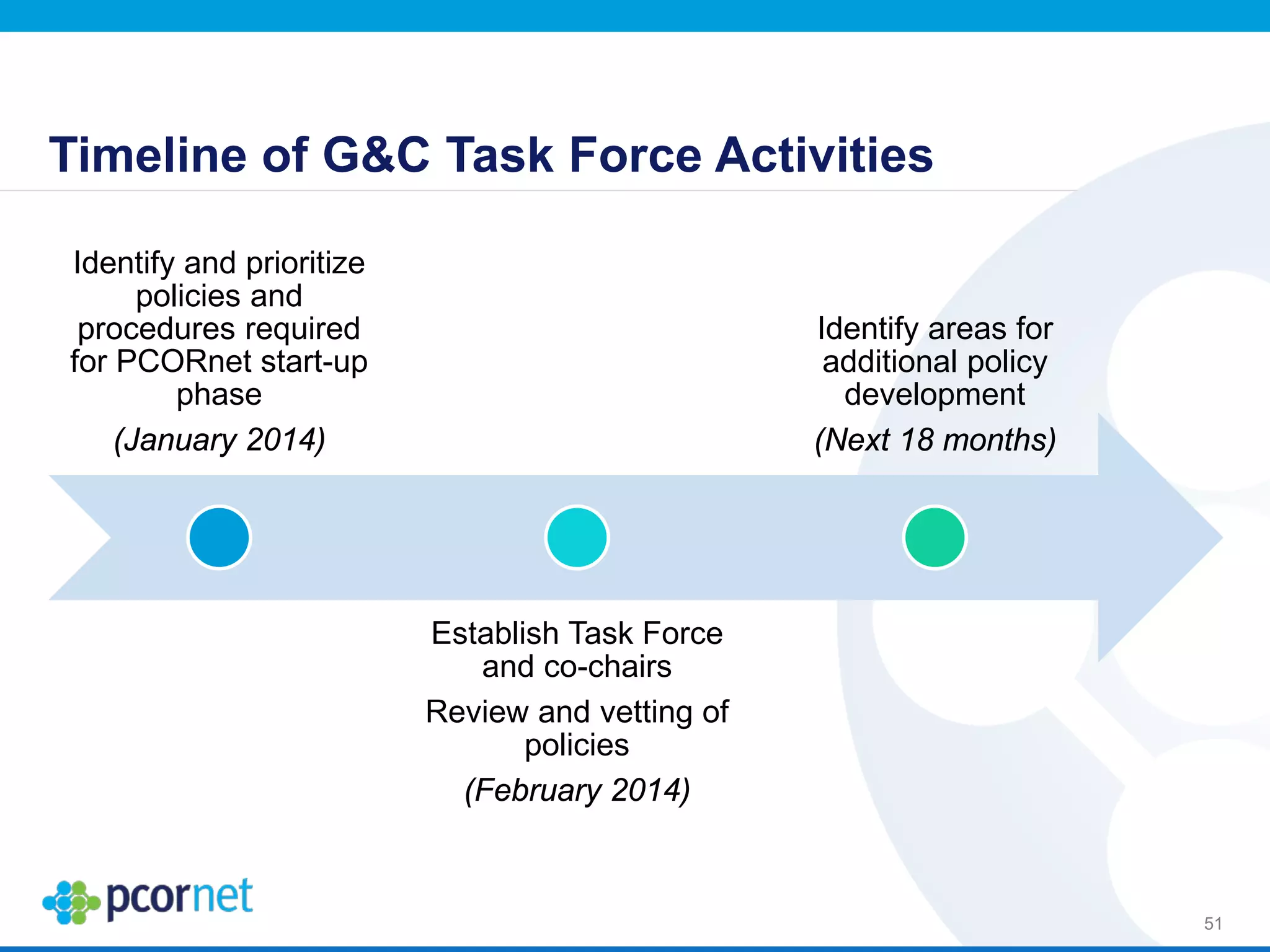
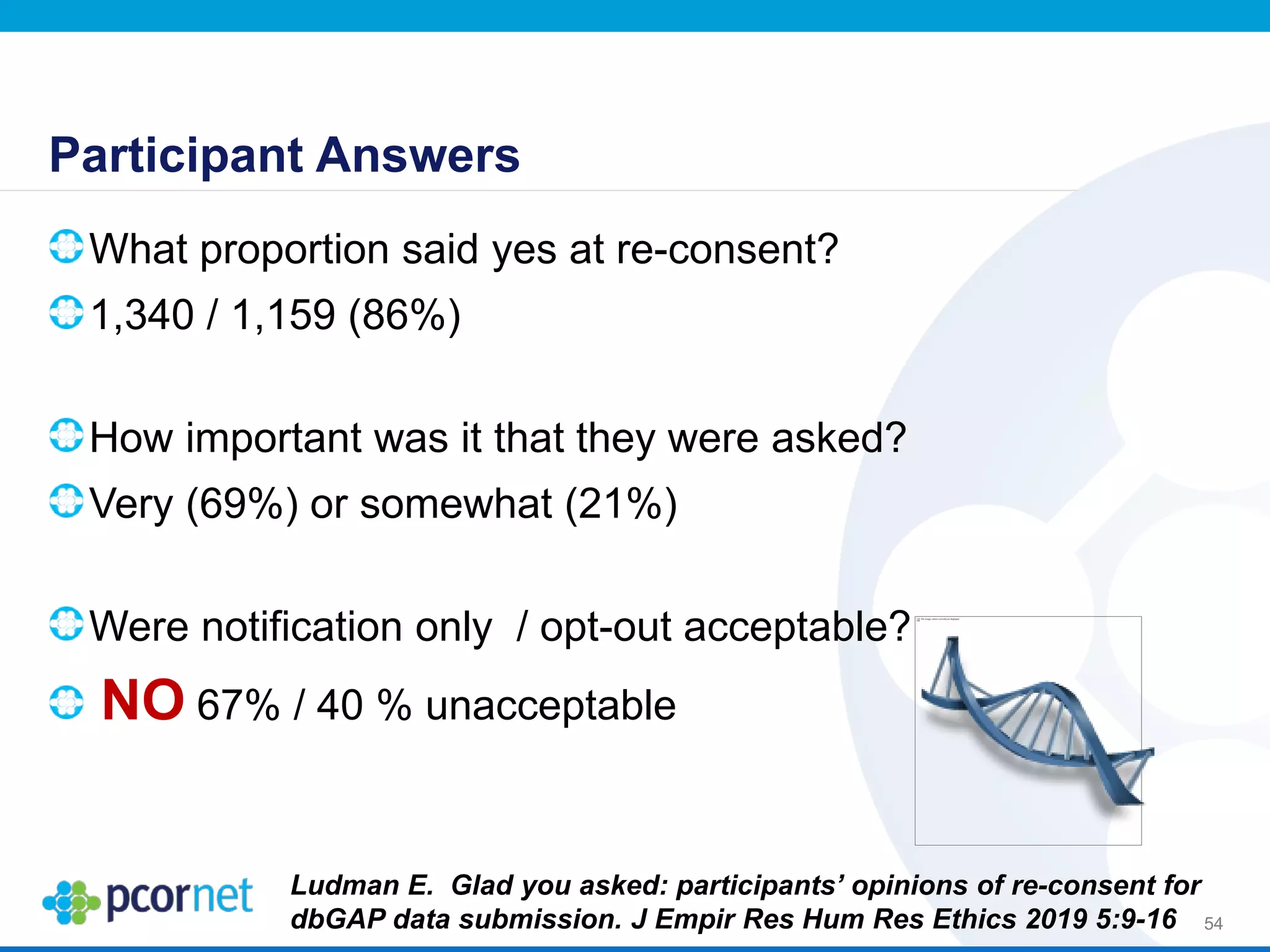
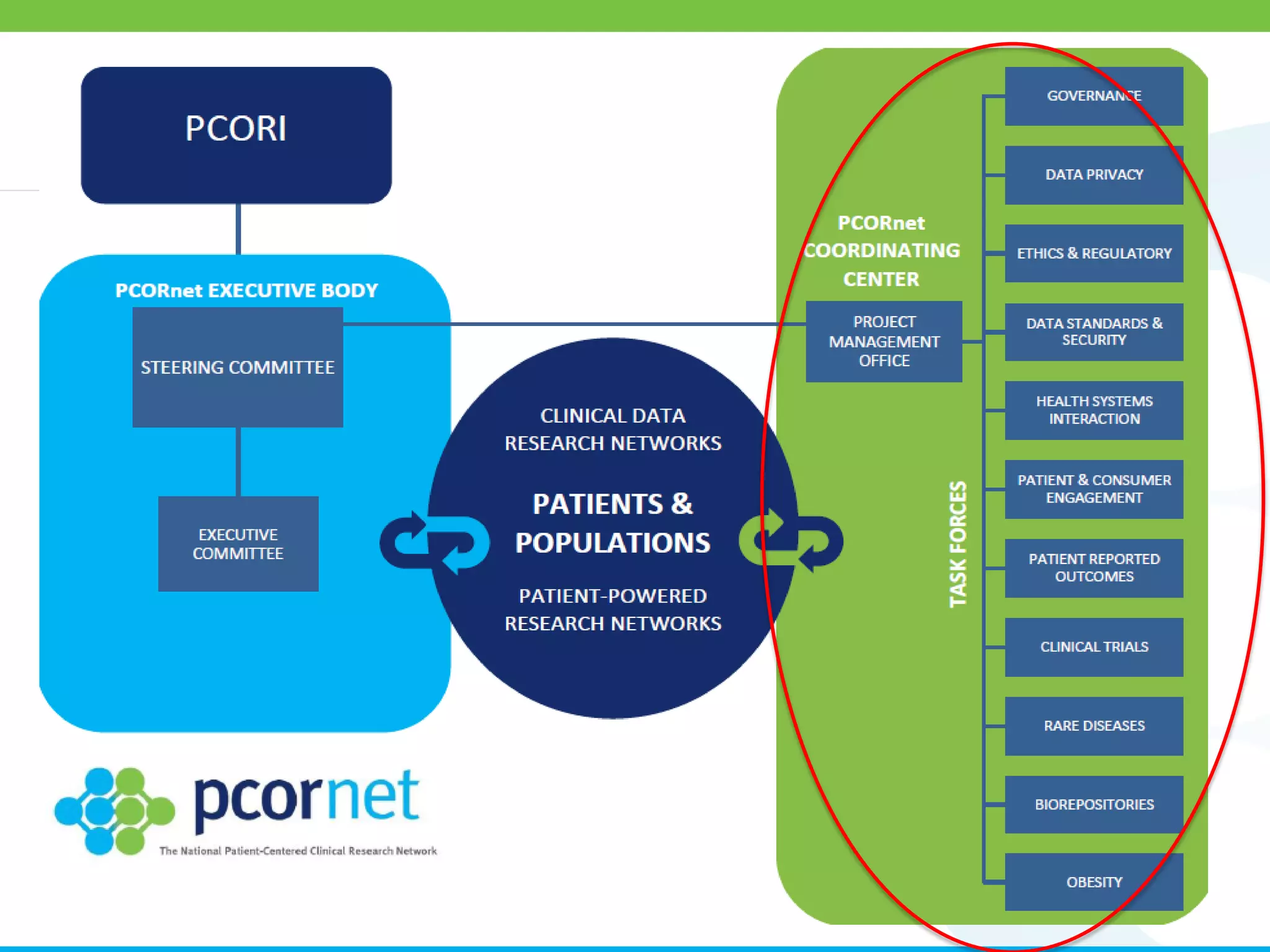
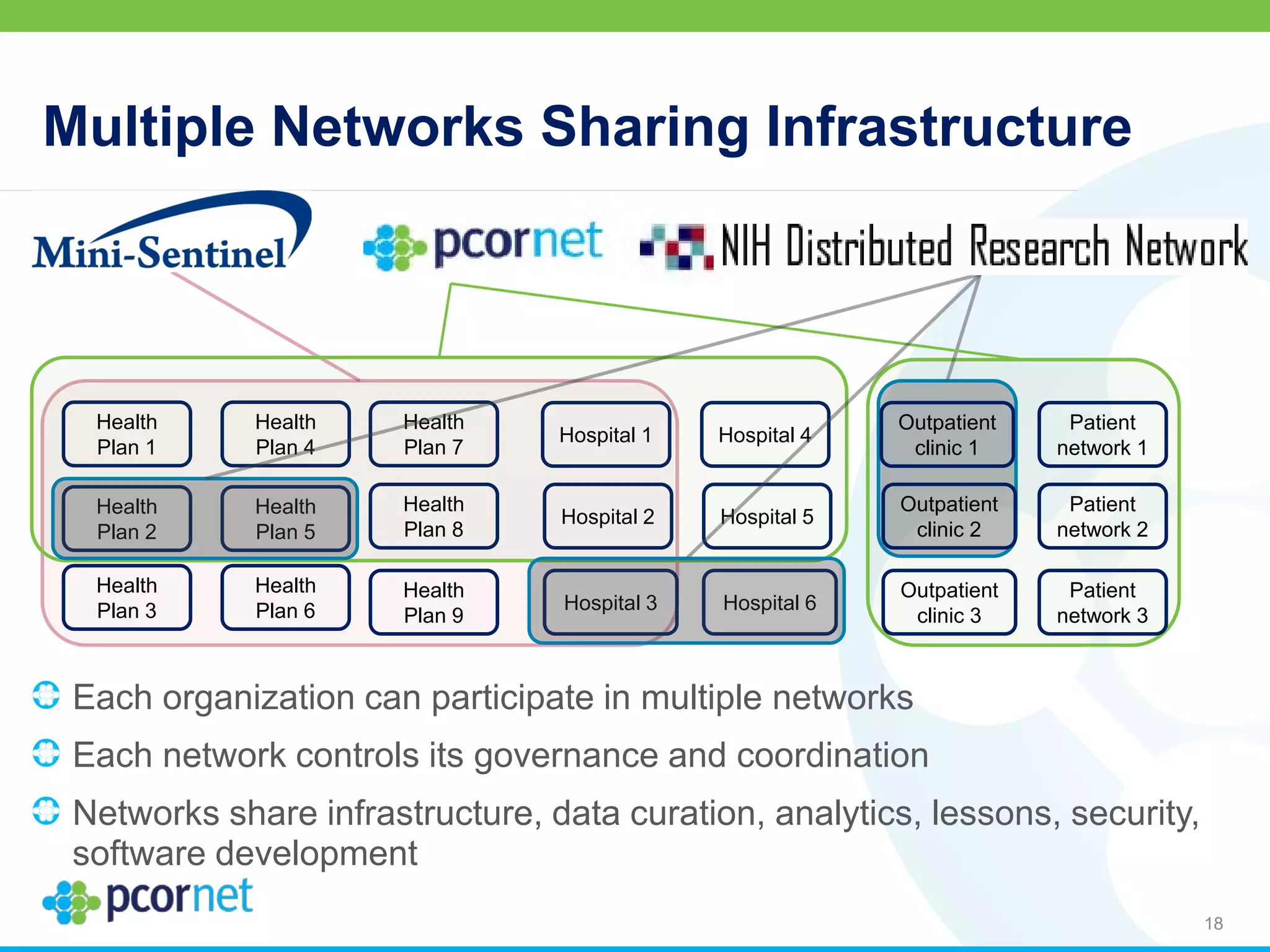
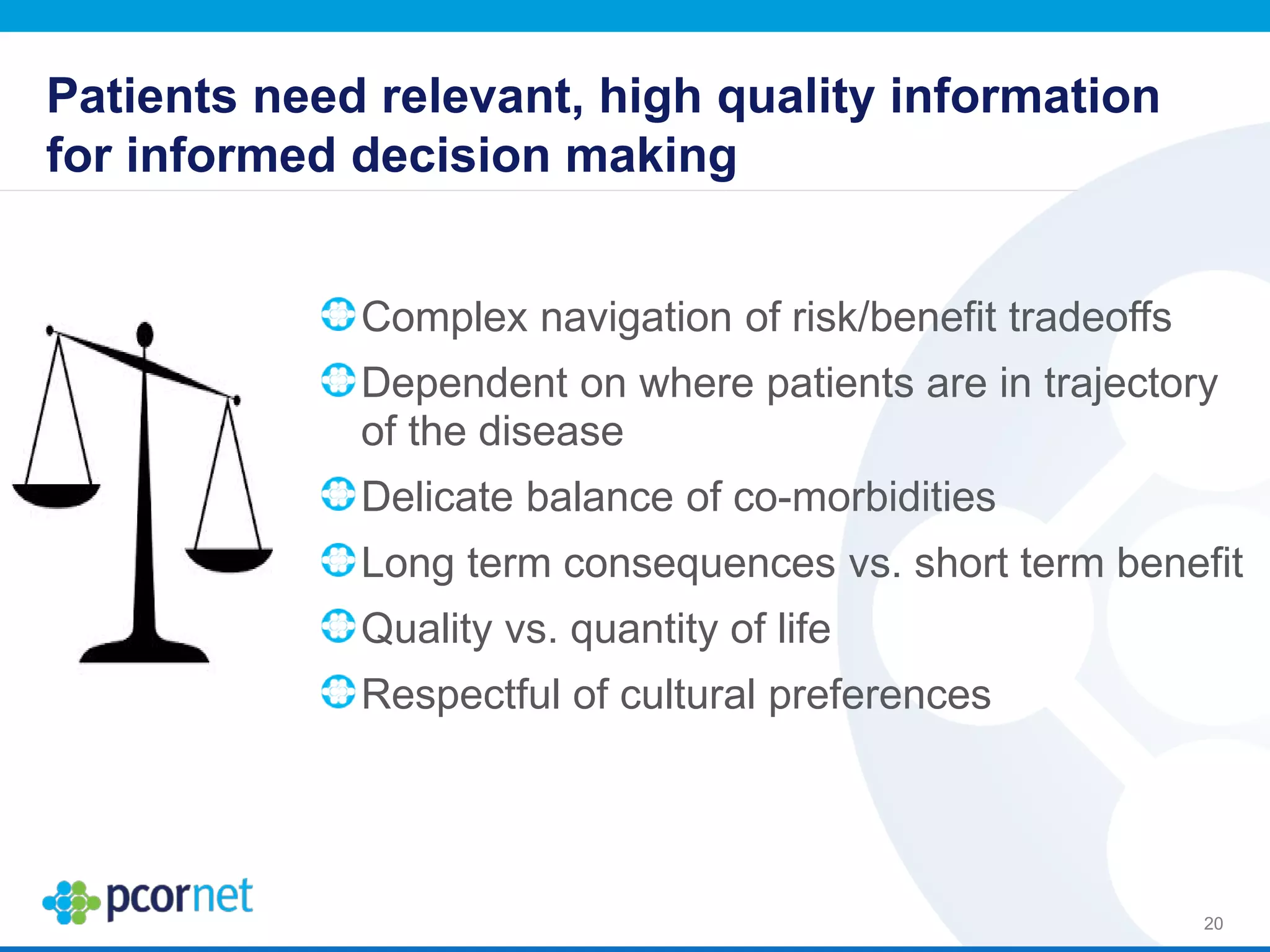
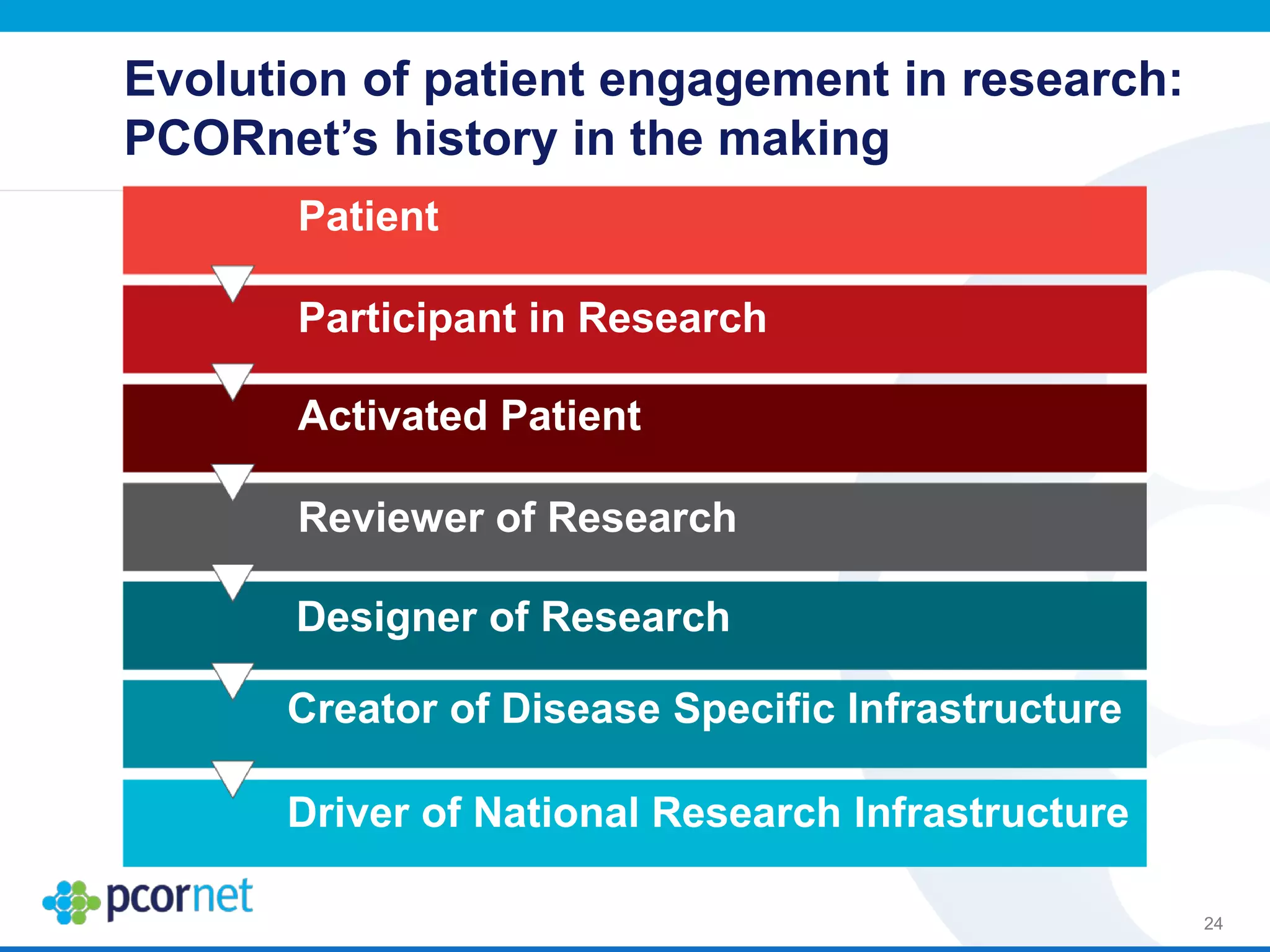
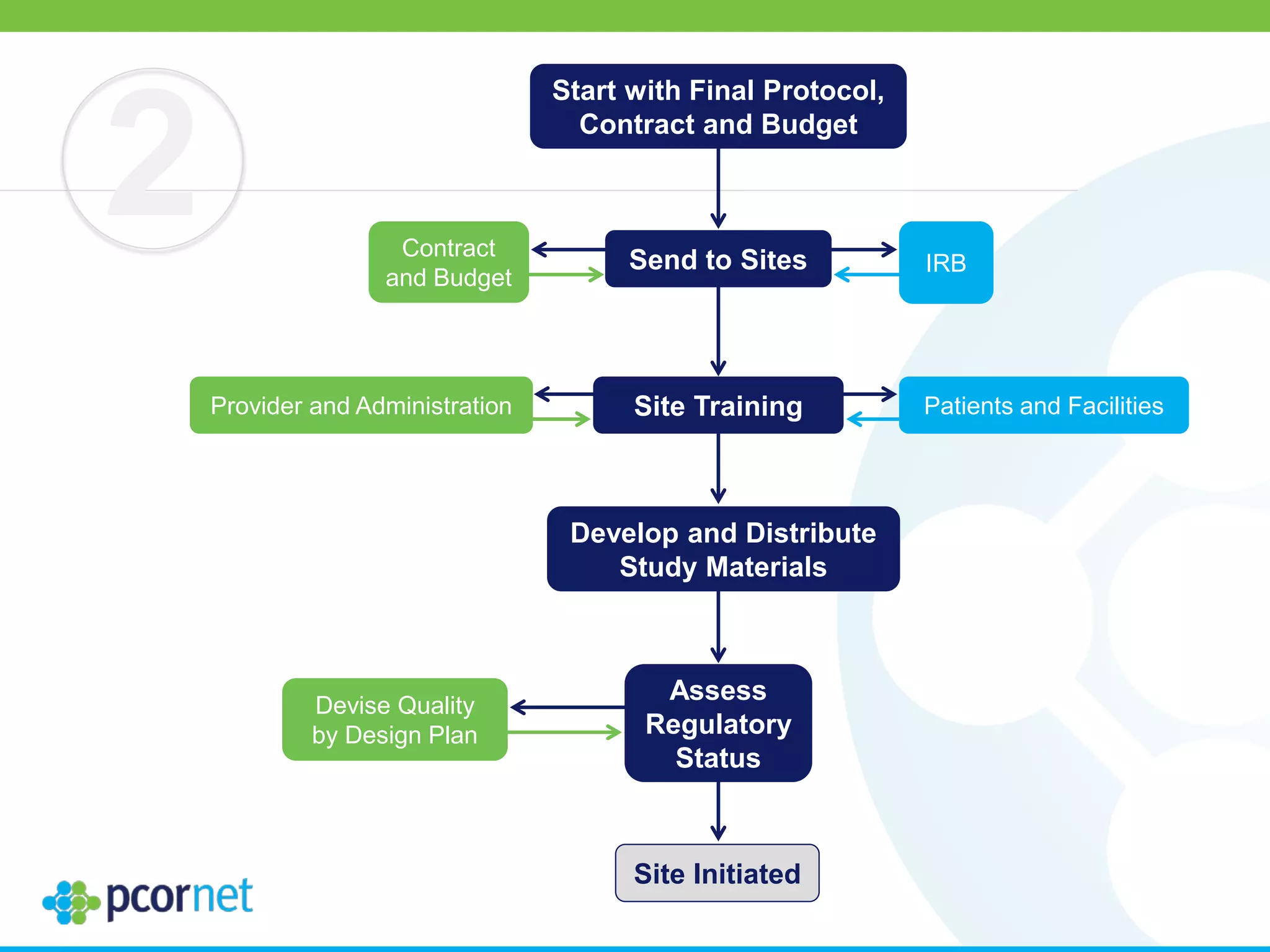
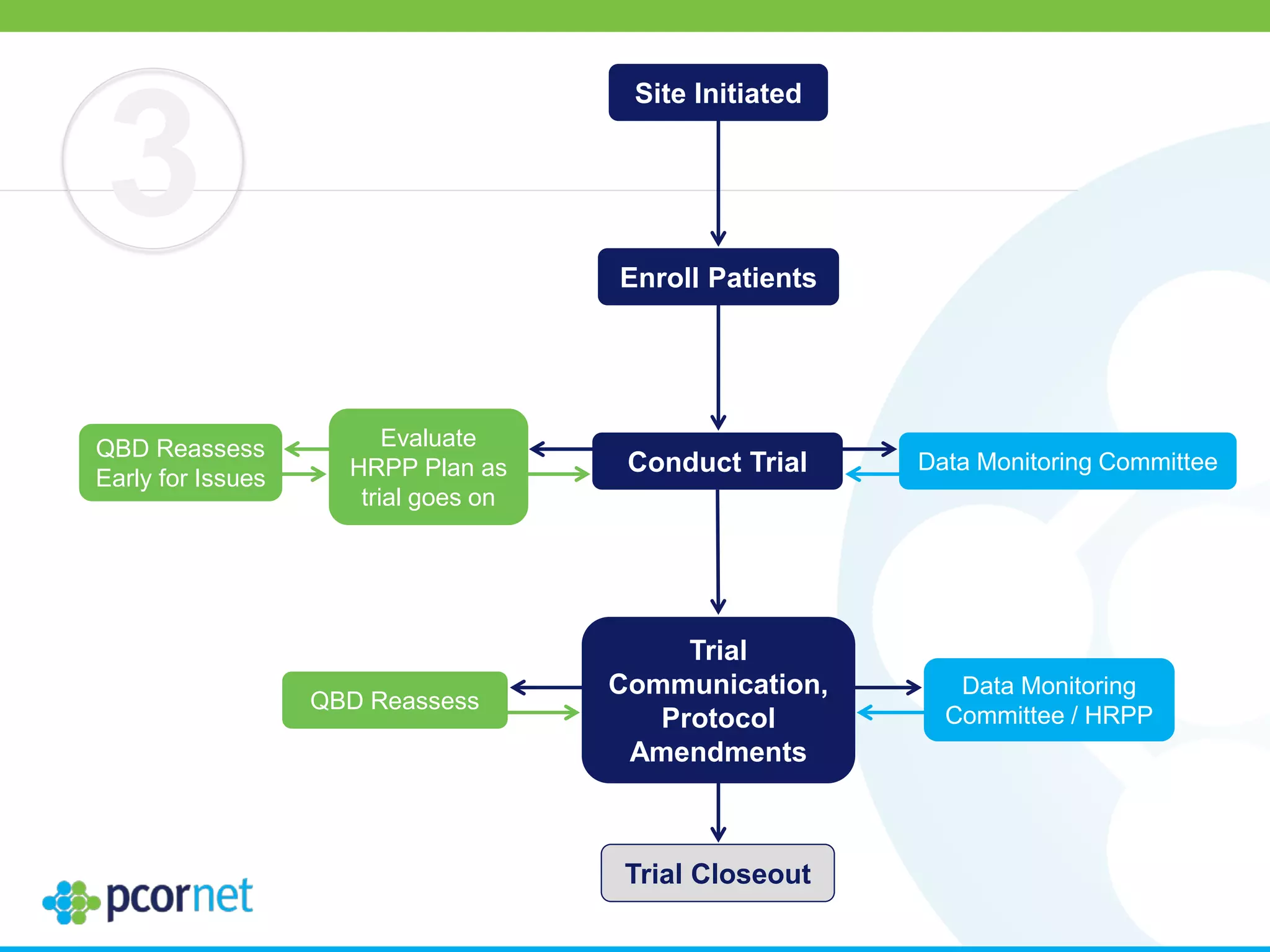
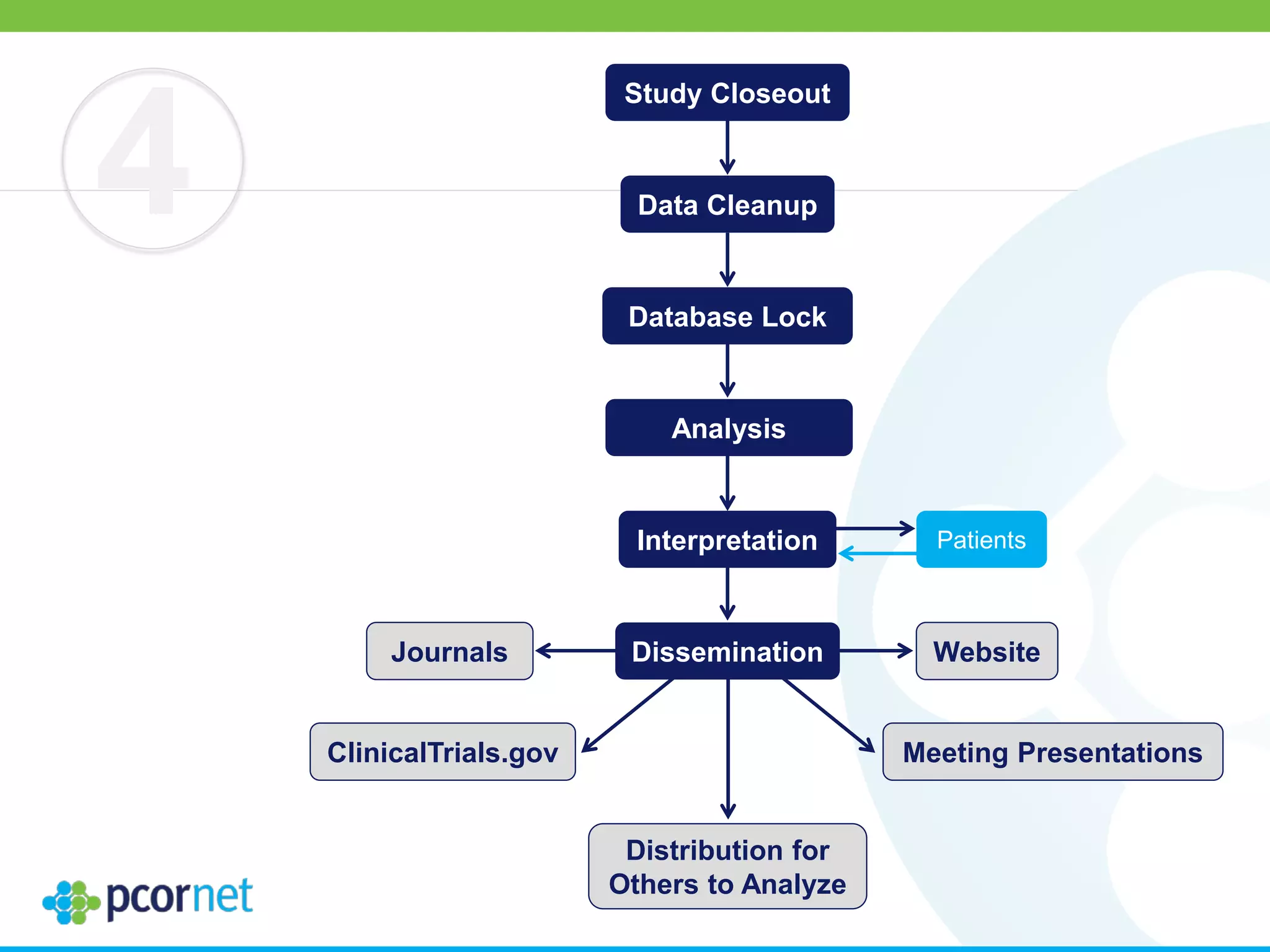
The document outlines the kickoff meeting agenda for PCORnet, a national patient-centered clinical research network, held on January 21-22, 2014, at the Brookings Institution. It emphasizes the collaboration of multiple healthcare networks to improve clinical outcomes research and engage patients, providers, and stakeholders. The agenda includes presentations on network governance, patient engagement, and building research capacities to better support healthcare decision-making.
























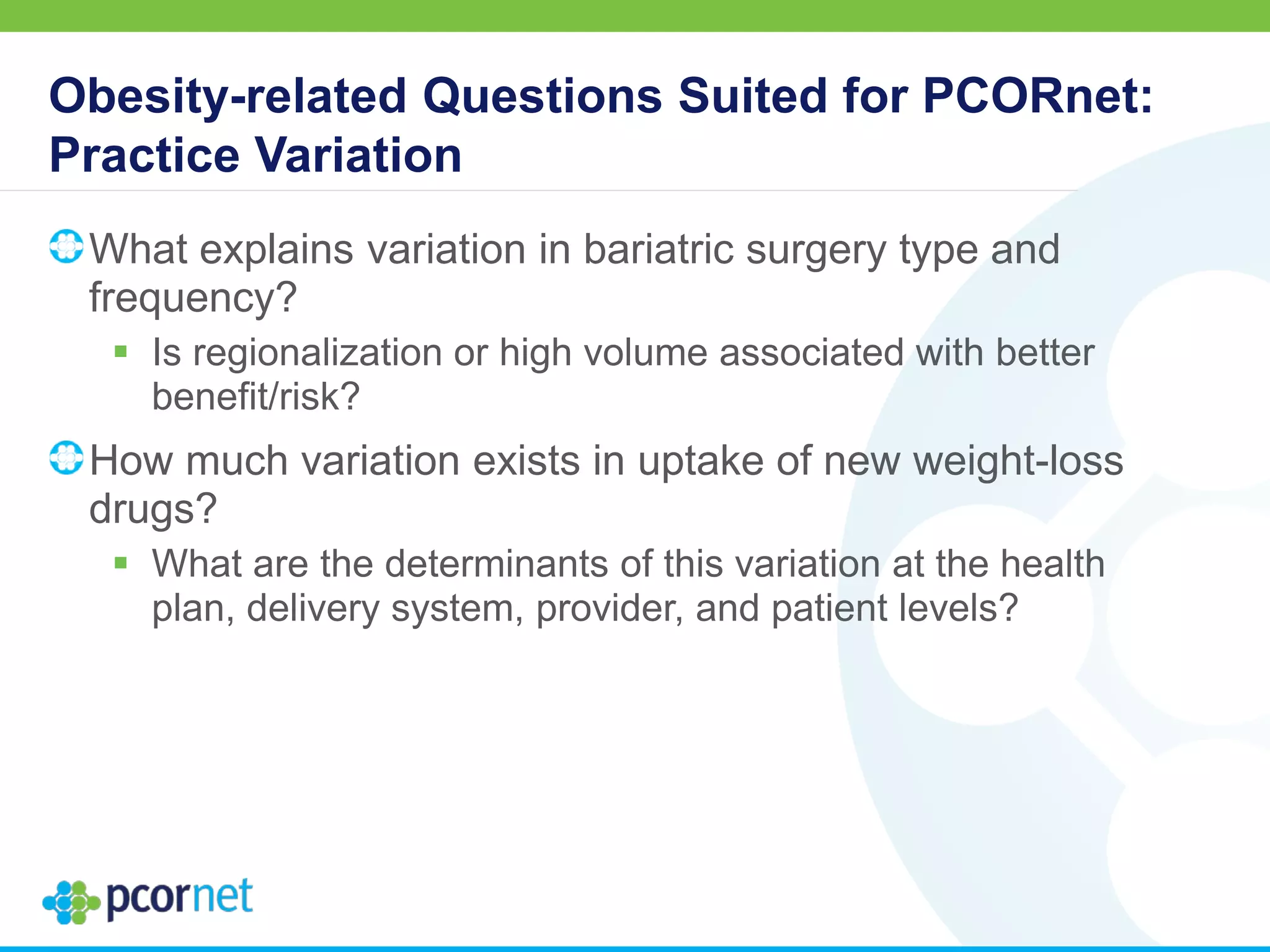

![Obesity-related Questions Suited for PCORnet:
Clinical Trials
Cluster randomized controlled trial
EMR-based identification, monitoring, evaluation, referral
and knowledge transfer
• E.g., to moderate excessive gestational weight gain
• E.g, to treat obesity in school age children
• [EpicCare has created tools for each of these examples]
Individual randomized controlled trial
Comparative effectiveness trial of the 2 newly approved
drugs for obesity treatment in adults: lorcaserin vs.
phentermine/topiramate
• Weight loss, improvements in metabolic parameters
• Adverse events, toxicity](https://image.slidesharecdn.com/pcori-pcornet-building-evidence-through-innovation-and-collaboration-0121141-140402133712-phpapp01/75/PCORnet-Building-Evidence-through-Innovation-and-Collaboration-43-2048.jpg)