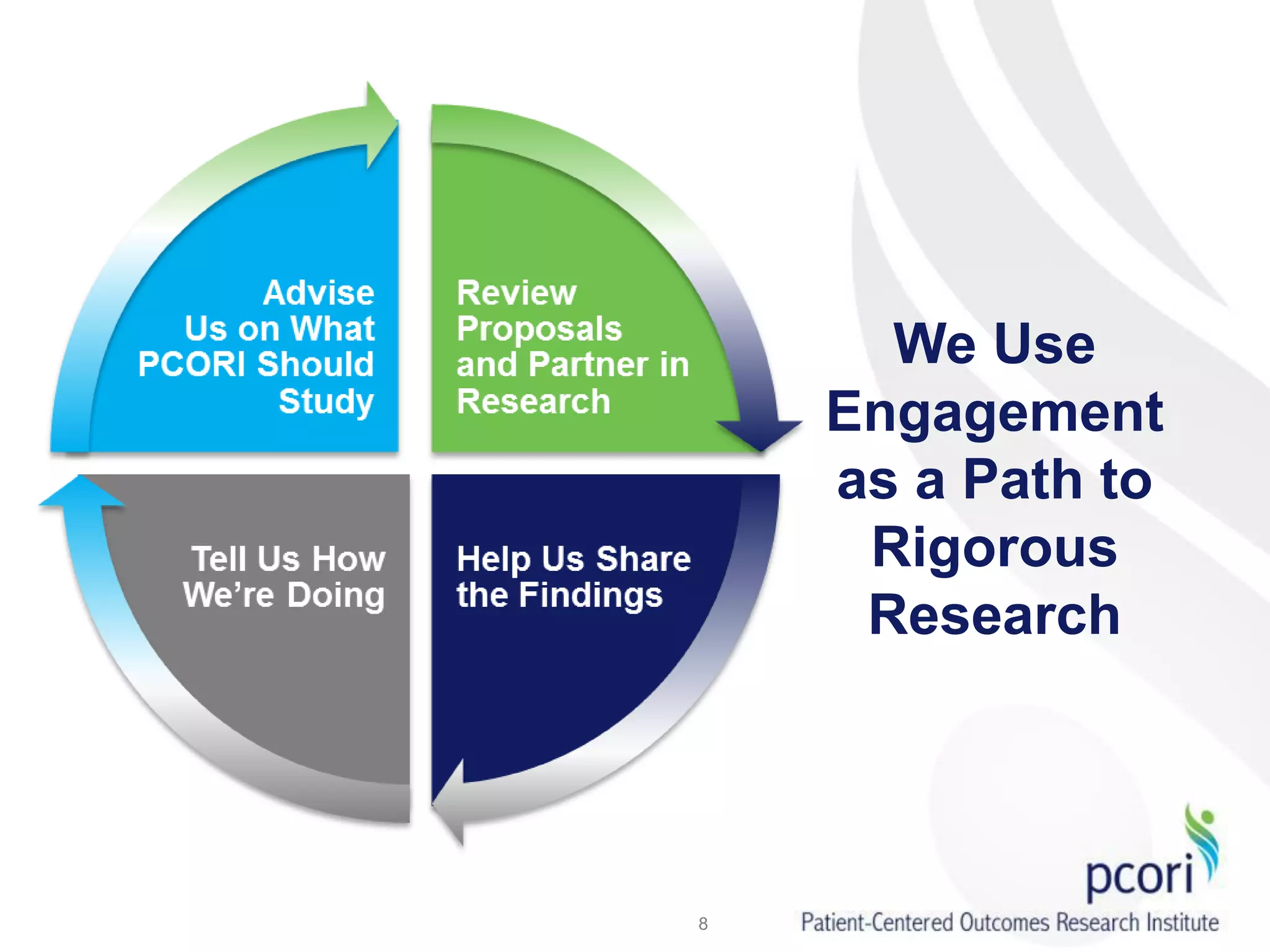
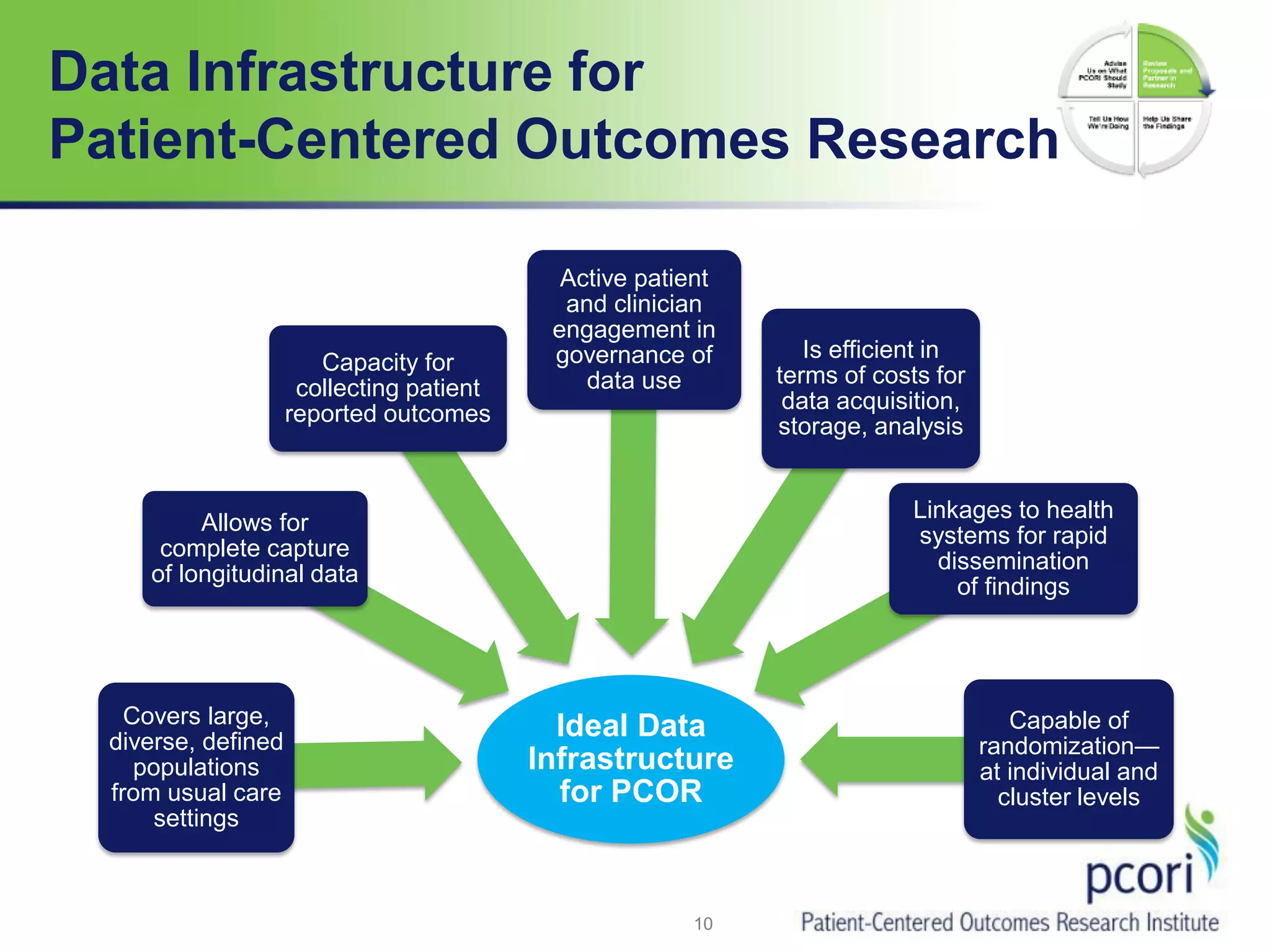
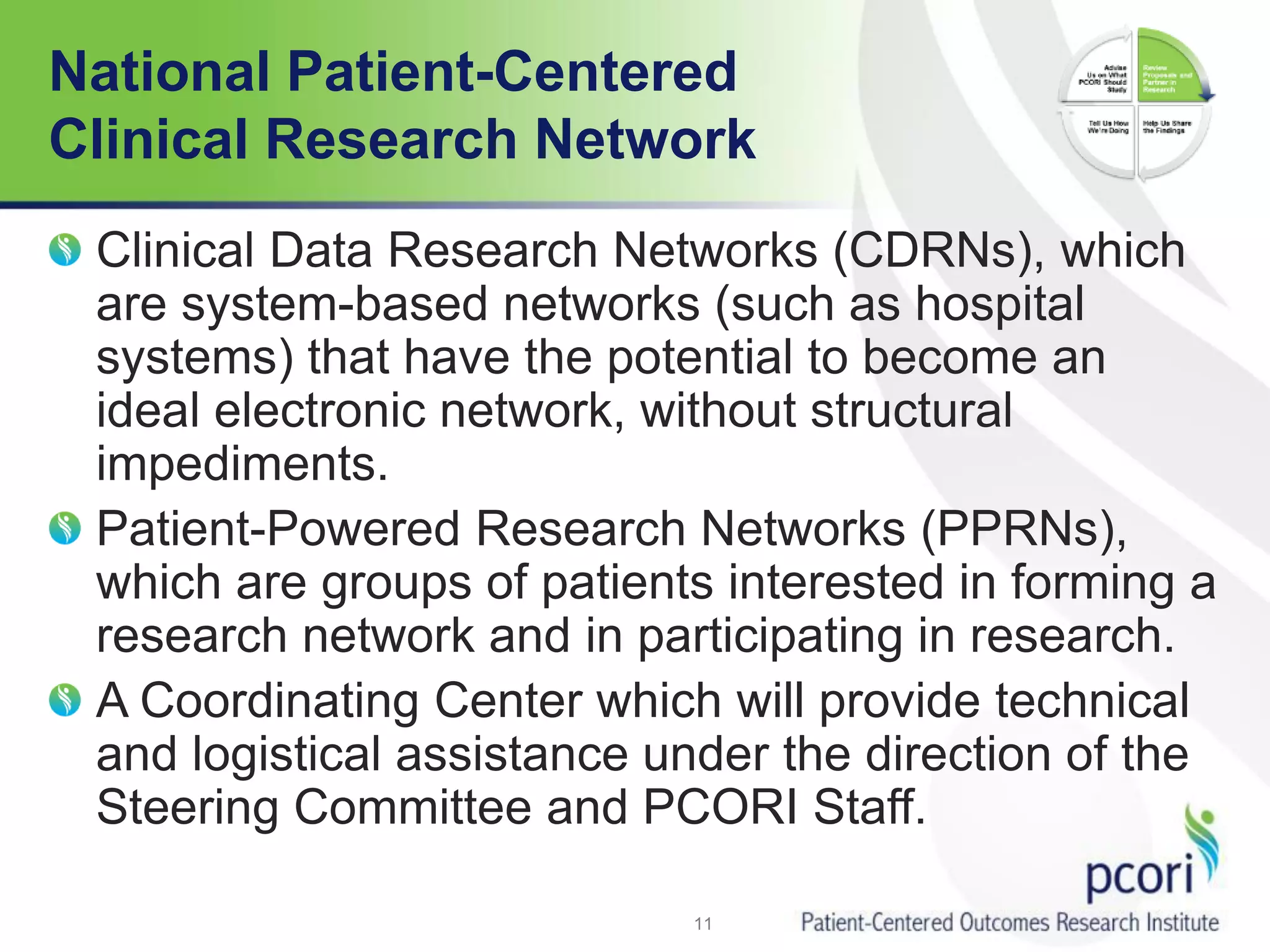
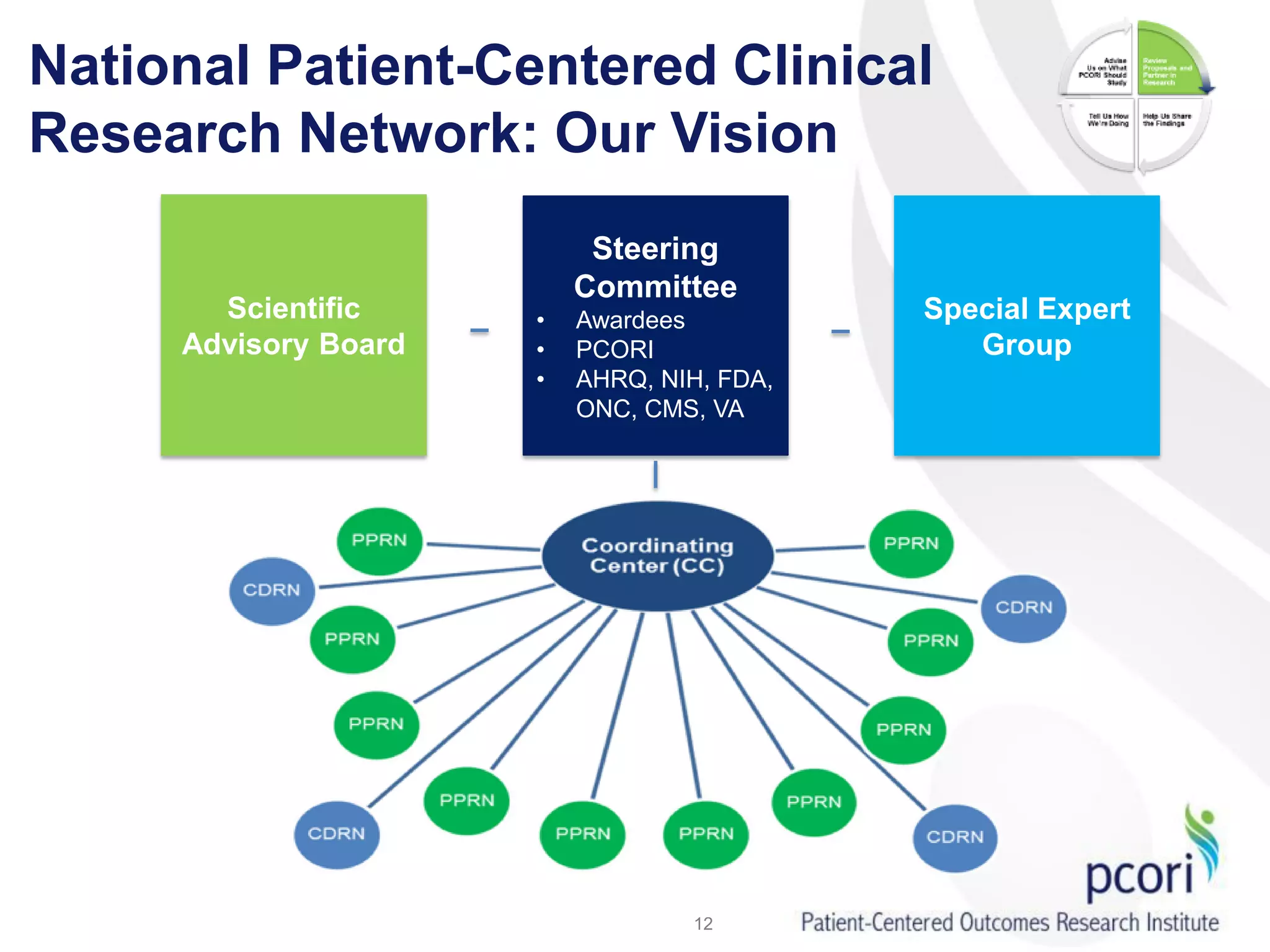
PCORI helps people make informed health care decisions by funding comparative effectiveness research guided by patients and caregivers. It focuses on improving outcomes for various subpopulations through research on prevention, diagnosis, treatment and healthcare systems. PCORI is establishing a National Patient-Centered Clinical Research Network involving clinical research networks, patient groups, and other partners to facilitate large-scale, real-world research.