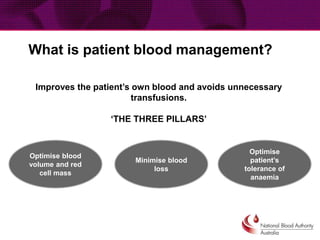
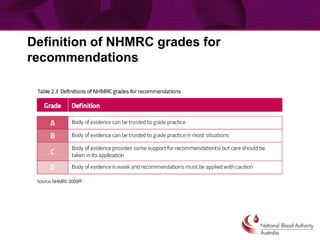
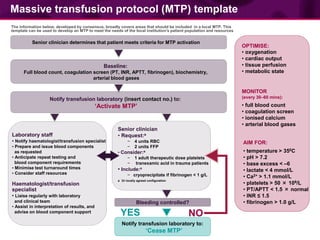
This document provides guidance on patient blood management and developing a massive transfusion protocol. It discusses three pillars of patient blood management: minimizing blood loss, optimizing blood volume and red cell mass, and optimizing patient tolerance of anemia. It recommends that in patients with critical bleeding requiring massive transfusion, red blood cell transfusions alone do not improve outcomes and that a protocol using specific ratios and timing of blood component therapies is needed. It provides a template for a massive transfusion protocol including criteria for activation, initial management, resuscitation targets, and special clinical considerations.