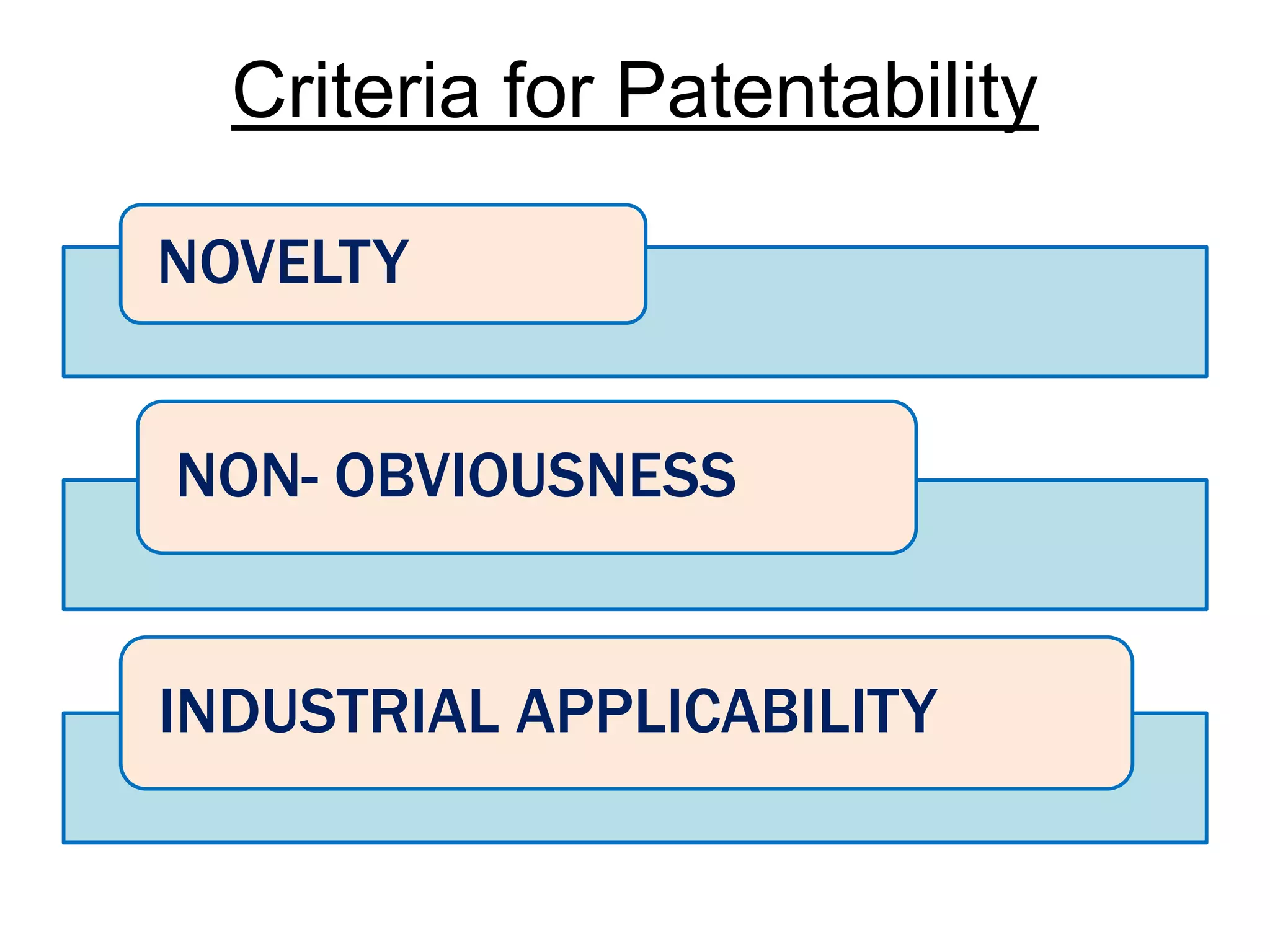
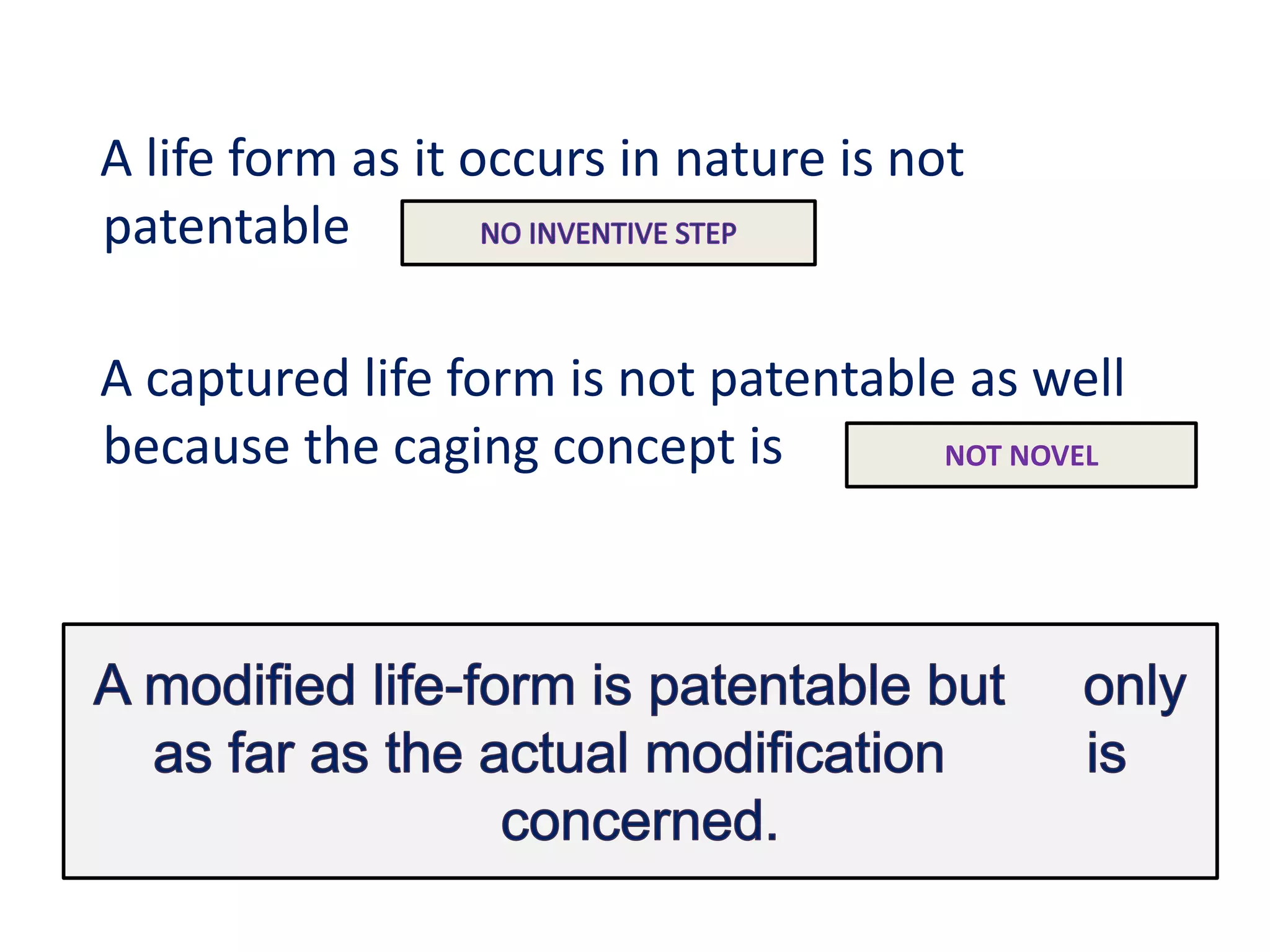
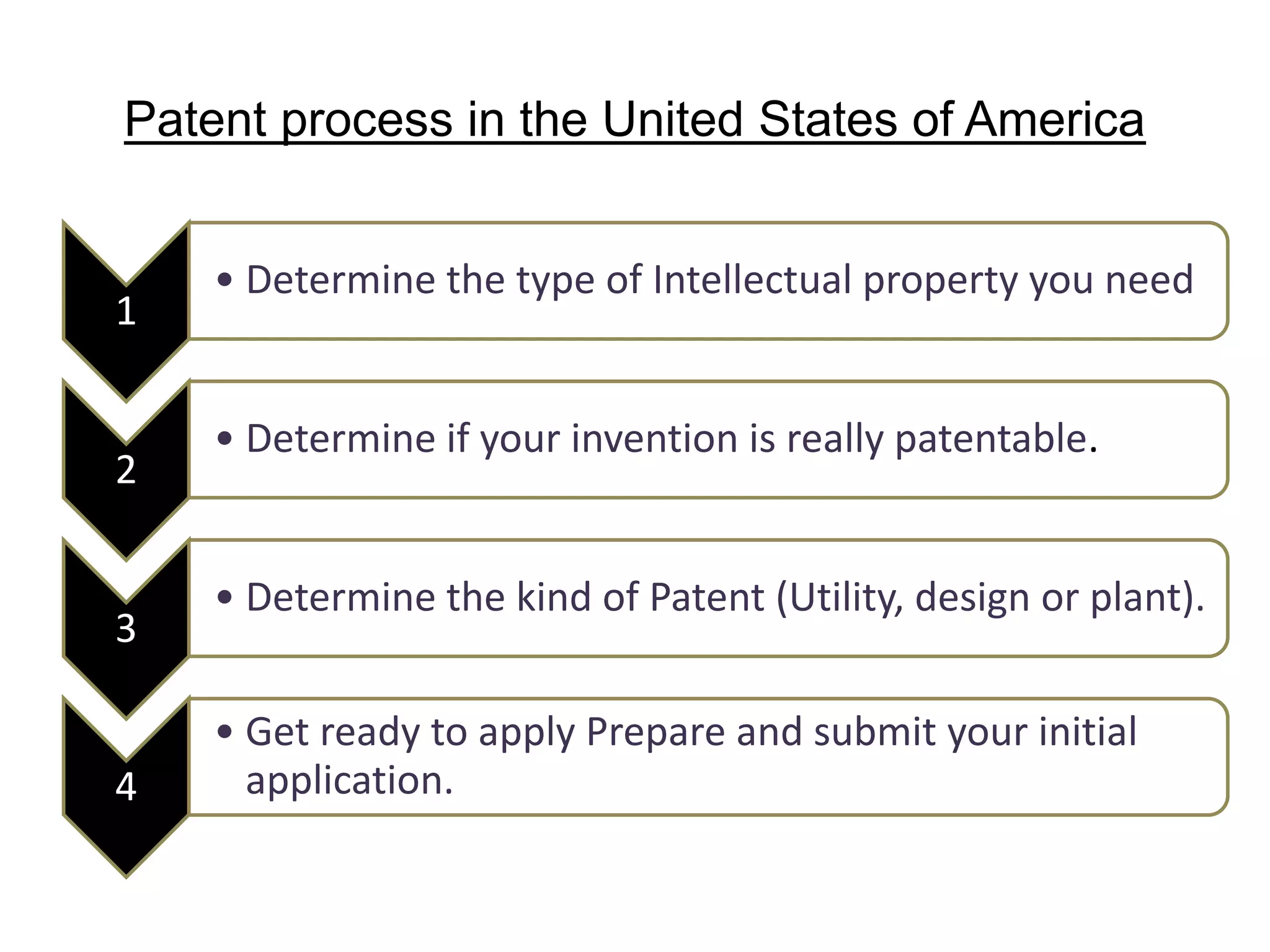
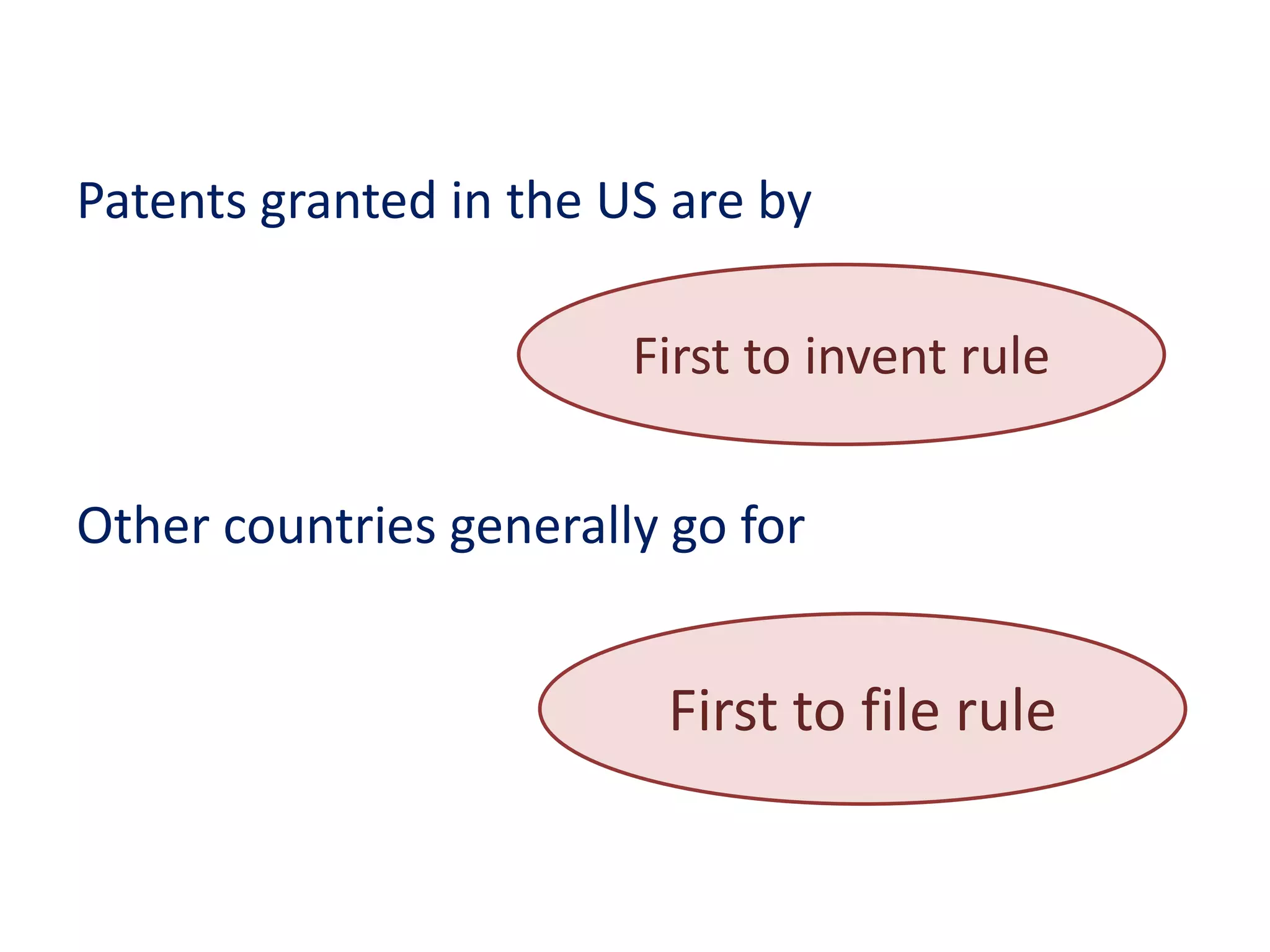
The document discusses the complex landscape of patenting biological life forms, emphasizing that naturally occurring life forms and essential biological processes are generally not patentable under international regulations like TRIPS. It highlights the historical context of biological patents starting from a 1980 US Supreme Court ruling and outlines the specific rules and limitations in both the US and India regarding patenting microorganisms and genetically modified organisms. Ethical concerns and the impact of patent ownership on agriculture and natural biodiversity are also raised, particularly in relation to the potential consequences of patenting life.