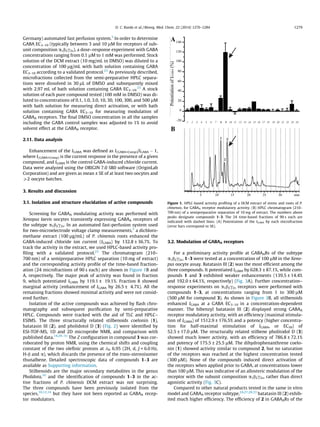
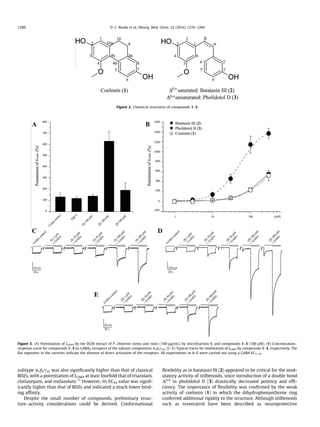
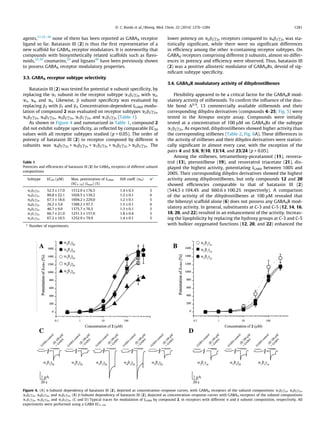
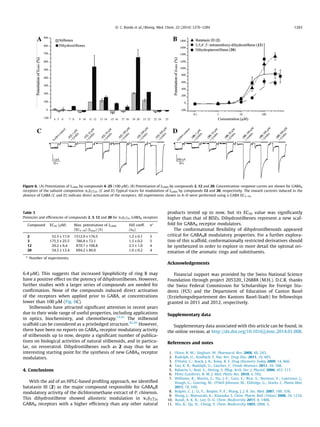
This study identified three dihydrostilbenoids (coelonin, batatasin III, and pholidotol D) from Pholidota chinensis that enhance GABA-induced chloride currents when tested on Xenopus laevis oocytes expressing GABAA receptors. Batatasin III showed the highest activity, enhancing currents by over 1500% at 300 μM, with an EC50 of 52.51 μM. A series of commercially available stilbenes and their dihydro derivatives were also tested, with the dihydro derivatives showing higher activity than the stilbenes. Dihydrostilbenes represent a new scaffold for GABAA receptor modulators.


![(17 mg) of fraction 13 (25 mg) afforded compounds 1 (2.3 mg) and
2 (6.5 mg). Compound 3 (2 mg) was isolated from 10 mg of fraction
14 (16 mg).
Compounds 1–3 were identified by comparison of their physio-
chemical data (NMR, ESI-TOFMS, and UV–vis) with published val-
ues.14,29–31
The purity was >95% (purity check by 1
H NMR).
2.5.1. Coelonin (1)
1
H NMR (methanol-d4, 500.13 MHz) dH (ppm): 8.13 (1H, d,
J = 8.4 Hz, H-5), 6.62 (1H, dd J = 8.3 and 2.7 Hz, H-6), 6.61 (1H, d,
J = 2.6 Hz, H-8), 6.30 (1H, d, J = 2.5 Hz, H-3), 6.26 (1H, d,
J = 2.5 Hz, H-1), 3.67 (3H, s, 4-OCH3), 2.59 (4H, s, H-9 and H-10);
13
C shifts (derived from multiplicity-edited HSQC and HMBC spec-
tra), dC (ppm): 158.3 (C, C-4), 155.4 (C, C-2), 154.8 (C, C-7), 139.8 (C,
C-8a), 138.7 (C, C-10a), 128.6 (CH, C-5), 125.2 (C, C-4b), 114.8 (C,
C-4a), 113.8 (CH, C-8), 112.2 (CH, C-6), 104.8 (CH, C-1), 100.1
(CH, C-3), 54.2 (4-OCH3), 30.8 (CH2, C-10) 30.1 (CH2, C-9).
HR-ESIMS m/z 243.1016 [M+H]+
(calcd for C15H15O3, 243.1016).
2.5.2. Batatasin III (2)
1
H NMR (methanol-d4, 500.13 MHz) dH (ppm): 7.03 (1H, dd,
J = 7.9 and 7.5 Hz, H-50
), 6.64 (3H, m, H-20
, H-40
, and H-60
), 6.29
(1H, br s, H-2), 6.23 (2H, m, H-4 and H-6), 3.63 (3H, s, 5-OCH3),
2.75 (4H, m, H-a and H-b); 13
C shifts (derived from multiplicity-
edited HSQC and HMBC spectra), dC (ppm): 160.9 (C, C-5), 157.6
(C, C-3), 156.7 (C, C-30
), 144.9 (C, C-1), 143.3 (C, C-10
), 129.0 (CH,
C-50
), 119.8 (CH, C-60
), 115.3 (CH, C-20
), 112.4 (CH, C-40
), 108.1
(CH, C-2), 105.5 (CH, C-6), 98.7 (CH, C-4), 54.3 (CH3, 5-OCH3),
37.6 (CH2, C-b), 37.0 (CH2, C-a). HR-ESIMS m/z 245.1176 [M+H]+
(calcd for C15H17O3, 245.1172).
2.5.3. Pholidotol D (3)
1
H NMR (methanol-d4, 500.13 MHz) dH (ppm): 7.17 (1H, dd,
J = 7.9 and 7.8 Hz, H-50
), 7.00–6.95 (4H, m, H-20
, H-60
, H-a and H-
b), 6.69 (1H, dd, J = 8.2 and 2.2 Hz, H-40
), 6.58 (2H, m, H-2 and H-
6), 6.31 (1H, t, J = 2 Hz, H-4), 3.76 (3H, s, 5-OCH3); 13
C shifts (de-
rived from multiplicity-edited HSQC and HMBC spectra), dC
(ppm): 160.8 (C, C-5), 157.7 (C, C-3), 156.0 (C, C-30
), 139.7 (C, C-
1), 138.5 (C, C-10
), 129.4 (CH, C-50
), 128.6 (CH, C-b), 128.4 (CH, C-
a), 117.8 (CH, C-60
), 114.4 (CH, C-40
), 112.4 (CH, C-20
), 105.8 (CH,
C-2), 103.4 (CH, C-6), 100.3 (CH, C-4), 54.4 (CH3, 5-OCH3). HR-
ESIMS m/z 243.1017 [M+H]+
(calcd for C15H15O3, 243.1016).
Further purification of compound 2 for subunit specificity tests
was achieved by separating a portion of the extract (7.3 g) by open
column chromatography (6 Â 69 cm, 700 g of silica gel), using a
step gradient of n-hexane–EtOAc (100:0, 95:5, 90:10, 85:15,
80:20, 75:25, 70:30, 65:35, 60:40, 55:45, 50:50, 40:60, 30:70,
20:80, 10:90, 0:100, 1 L each) and washing in the end with MeOH
100% (1.5 L). The flow rate was ca. 50 mL/min. The effluent was
combined to 15 fractions (1–15) based on TLC patterns. After
HPLC–PDA–MS analysis, fractions 7 and 8 were selected for isola-
tion of compound 2 by preparative HPLC, with acetonitrile (solvent
A) and water (solvent B), using a gradient from 40% A to 50% A in
30 min, followed by 50% A to 100% A in 5 min, hold for 10 min.
The flow rate was 20 mL/min. Stock solutions in THF (100 mg/
mL) were prepared and repeatedly injected in portions of 300–
400 lL. The separation of fractions 7 (129 mg) and 8 (132 mg),
yielded compound 10.8 mg of 2.
2.6. Synthesis of dihydrostilbenes
2.6.1. Stilbenes
Compounds 4–7, 9, 11, 13, 15, 17, and 19 were purchased from
TCI Europe N.V. Compounds 21 and 25 were purchased from Sig-
ma–Aldrich Co. Compound 23 was purchased from Santa Cruz Bio-
technology, Inc.
2.6.2. General procedure
Dihydro derivatives of compounds 7, 9, 11, 13, 15, 17, 19, 21,
and 23 were prepared by hydrogenation of corresponding stilb-
enes. A standard protocol was followed,32
with minor modifica-
tions. Solutions of each stilbene (10 mg) in absolute EtOH (5 ml)
were stirred under H2 for 3 h in the presence of 10% Pd/C. The reac-
tion mixtures were filtered over Celite to remove the catalyst, and
evaporated to dryness. The resulting residues were purified by
flash column chromatography, using a hexane/EtOAc gradient, to
afford target compounds 8, 10, 12, 14, 16, 18, 20, 22, and 24,
respectively, in yields of 85–95%. The spectroscopic data of com-
pounds were in agreement with the literature, except for com-
pound 24, for which no report was found (1
H NMR spectrum is
provided as Supporting information).32–41
2.6.3. trans-2-Fluoro-40
-methoxy-dihydrostilbene (24)
1
H NMR (chloroform-d4, 500.13 MHz) dH (ppm): 7.26–7.08 (4H,
m), 7.08–7.98 (2H, m), 6.90–6.80 (2H, m), 3.81 (3H, s), 3.05–2.84
(4H, m). HRESI-MS m/z 253.1589 [M+Na]+
(calcd formula weight
for C15H15FO, 230.2774).
2.7. Expression of GABAA receptors
Stage V–VI oocytes from Xenopus laevis were prepared, and
cRNA injected as previously described.23
Female Xenopus laevis
(NASCO, Fort Atkinson, WI) were anesthetized by exposing them
for 15 min to a 0.2% MS-222 (methanesulfonate salt of 3-amino-
benzoic acid ethyl, Sigma) solution before surgically removing
parts of the ovaries. Follicle membranes from isolated oocytes were
enzymatically digested with 2 mg/mL collagenase from Clostridium
histolyticum (Type 1A, Sigma). Synthesis of capped runoff poly(A+)
cRNA transcripts was obtained from linearized cDNA templates
(pCMV vector). Directly after enzymatic isolation, the oocytes were
injected with 50 nL of DEPC-treated water (Sigma) containing dif-
ferent cRNAs at a concentration of approximately 300–3000 pg/nL
per subunit. The amount of injected cRNA mixture was determined
by means of a NanoDrop ND-1000 (Kisker Biotech). To ensure
expression of the gamma subunit in a1b2c2S receptors, rat cRNAs
were mixed in a 1:1:10 ratio. Oocytes were then stored at 18 °C
in ND96 solution containing 1% of penicillin–streptomycin solution
(Sigma–Aldrich). Voltage clamp measurements were performed
between days 1 and 5 after cRNA injection.
2.8. Positive control
Diazepam (7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-
benzodiazepin-2-one, Sigma, purity not less than 98%) was used
as positive control. At 1 lM diazepam enhanced IGABA up to
231.3 ± 22.6% (n = 3). See also Figure S1, Supporting information.
2.9. Two-microelectrode voltage clamp studies
Electrophysiological experiments were performed by the two-
microelectrode voltage clamp method making use of a TURBO
TEC 03X amplifier (npi electronic GmbH) at a holding potential
of À70 mV and pCLAMP 10 data acquisition software (Molecular
Devices). Currents were low-pass-filtered at 1 kHz and sampled
at 3 kHz. The bath solution contained 90 mM NaCl, 1 mM KCl,
1 mM MgCl2, 1 mM CaCl2, and 5 mM HEPES (pH 7.4). Electrode fill-
ing solution contained 2 M KCl. Oocytes with maximal current
amplitudes >3 lA were discarded to exclude voltage clamp errors.
2.10. Fast solution exchange during IGABA recordings
Test solutions (100 lL) were applied to the oocytes at a speed of
300 lL/s by means of the ScreeningTool (npi electronic, Tamm,
1278 D. C. Rueda et al. / Bioorg. Med. Chem. 22 (2014) 1276–1284](https://image.slidesharecdn.com/eeb1f115-e369-481e-9c79-600594d29c76-160528150824/85/Paper-3_2-3-320.jpg)