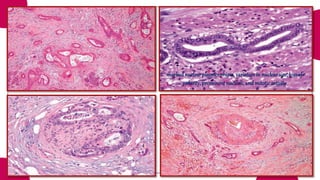
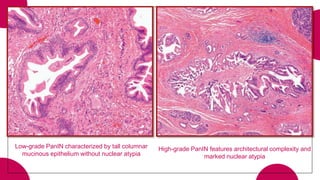
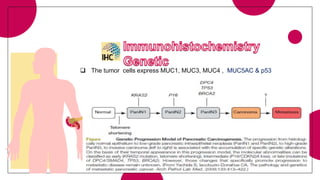
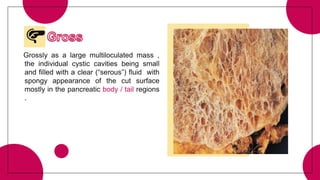
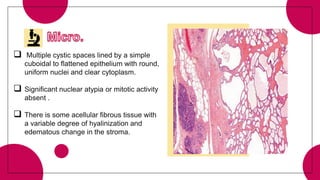
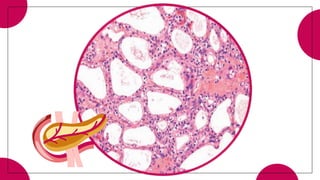
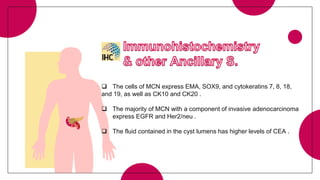
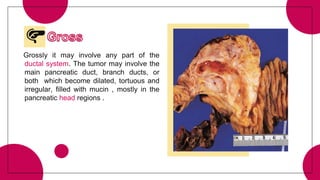
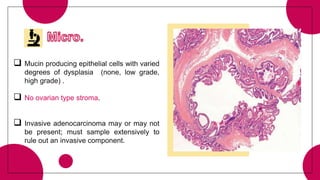
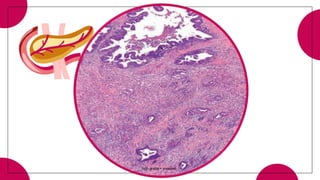
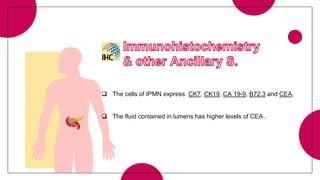
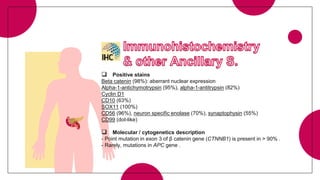
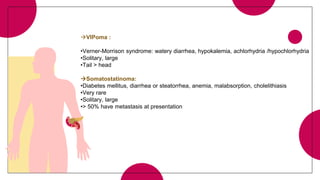
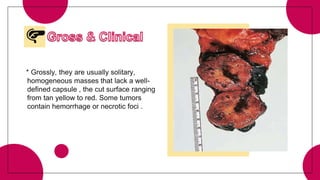
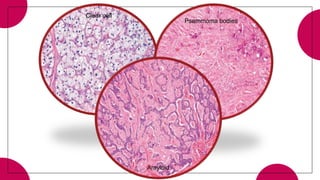
This document provides an overview of various types of pancreatic neoplasms, including:
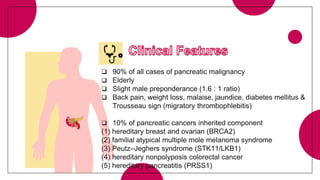
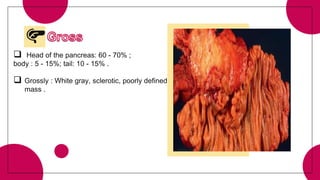
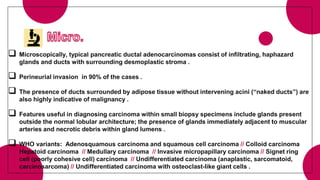
1. Ductal adenocarcinoma, which accounts for 90% of pancreatic cancers.
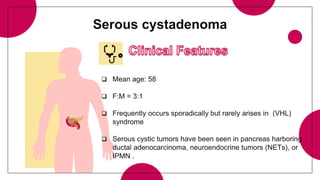
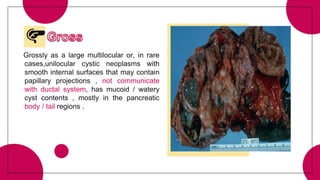
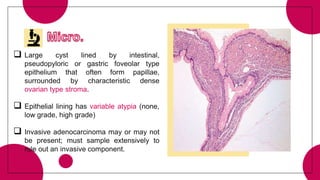
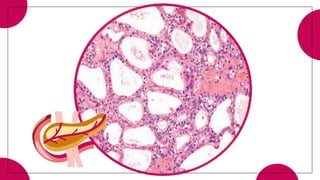
2. Cystic pancreatic neoplasms such as serous cystadenomas and mucinous cystic neoplasms.
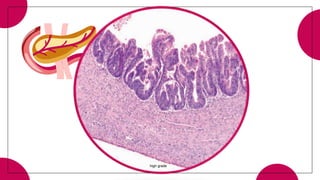
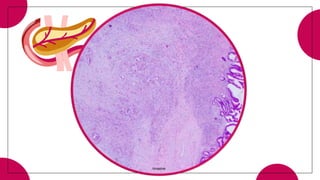
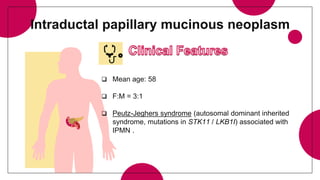
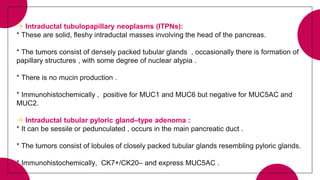
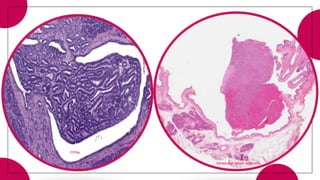
3. Intraductal neoplasms including intraductal papillary mucinous neoplasms and intraductal tubular neoplasms.
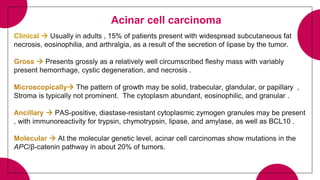
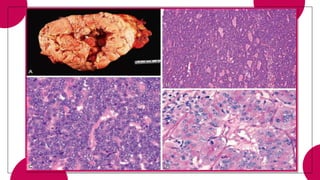
4. Acinar cell tumors, the most common being acinar cell carcinoma.
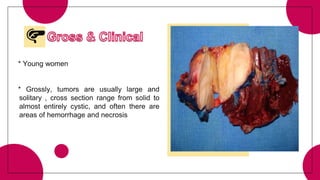
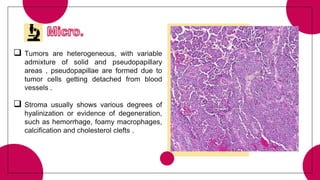
5. Other rare tumors such as pancreatoblastoma, solid pseudopapillary tumor, and pancreatic neuroendocrine tumors.
Detailed information