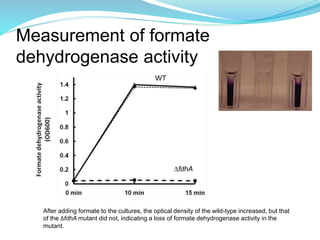
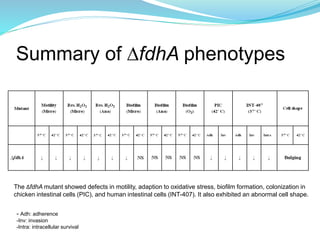
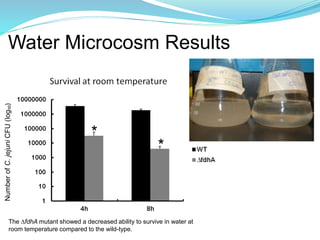
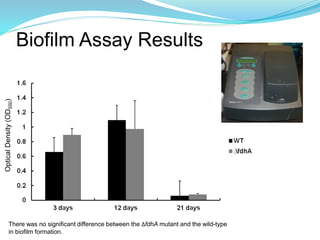
This study investigated the role of the formate dehydrogenase gene (fdhA) in the persistence of Campylobacter jejuni in water. The researcher hypothesized that a C. jejuni mutant strain lacking fdhA (ΔfdhA) would have a lower survival rate in water compared to the wild-type strain. Results showed that the ΔfdhA mutant had decreased ability to survive in water microcosms but did not differ in biofilm formation. Future studies could examine whether the ΔfdhA mutant enters the viable but non-culturable state more readily or is more susceptible to chemical factors in water.