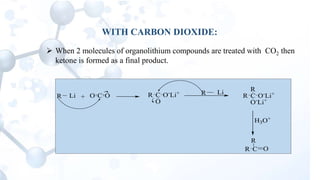
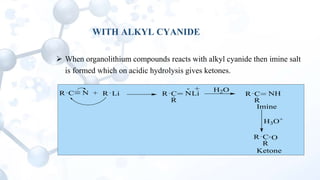
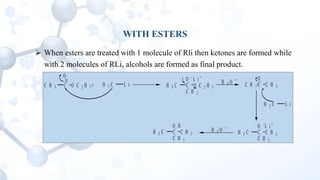
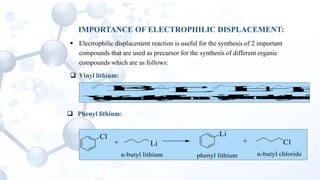
This presentation discusses the reactions of organolithium compounds. Organolithium compounds undergo several reactions including: reaction with carbon dioxide to form ketones; reaction with oxygen to form hydroperoxides; reaction with esters and alkyl cyanides to form ketones; and electrophilic displacement reactions with organic halides. Electrophilic displacement, or metal-halogen exchange, is an important reaction as it allows for the synthesis of reactive organolithium compounds like vinyl lithium and phenyl lithium which can be used as precursors in organic synthesis.