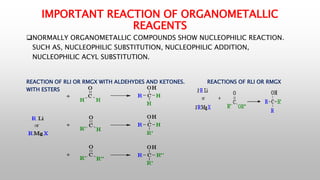
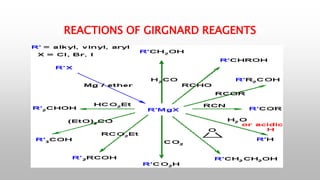
This document provides an introduction to organometallic compounds and reagents. It discusses that organometallic compounds contain carbon-metal bonds and some important examples. Organometallic reagents of magnesium, lithium, and copper are important for stoichiometric and industrial reactions. The document also summarizes key reactions of organolithium, Grignard, and organocopper reagents that involve nucleophilic addition or substitution. Finally, it recognizes three great contributors to the field of organometallic chemistry.