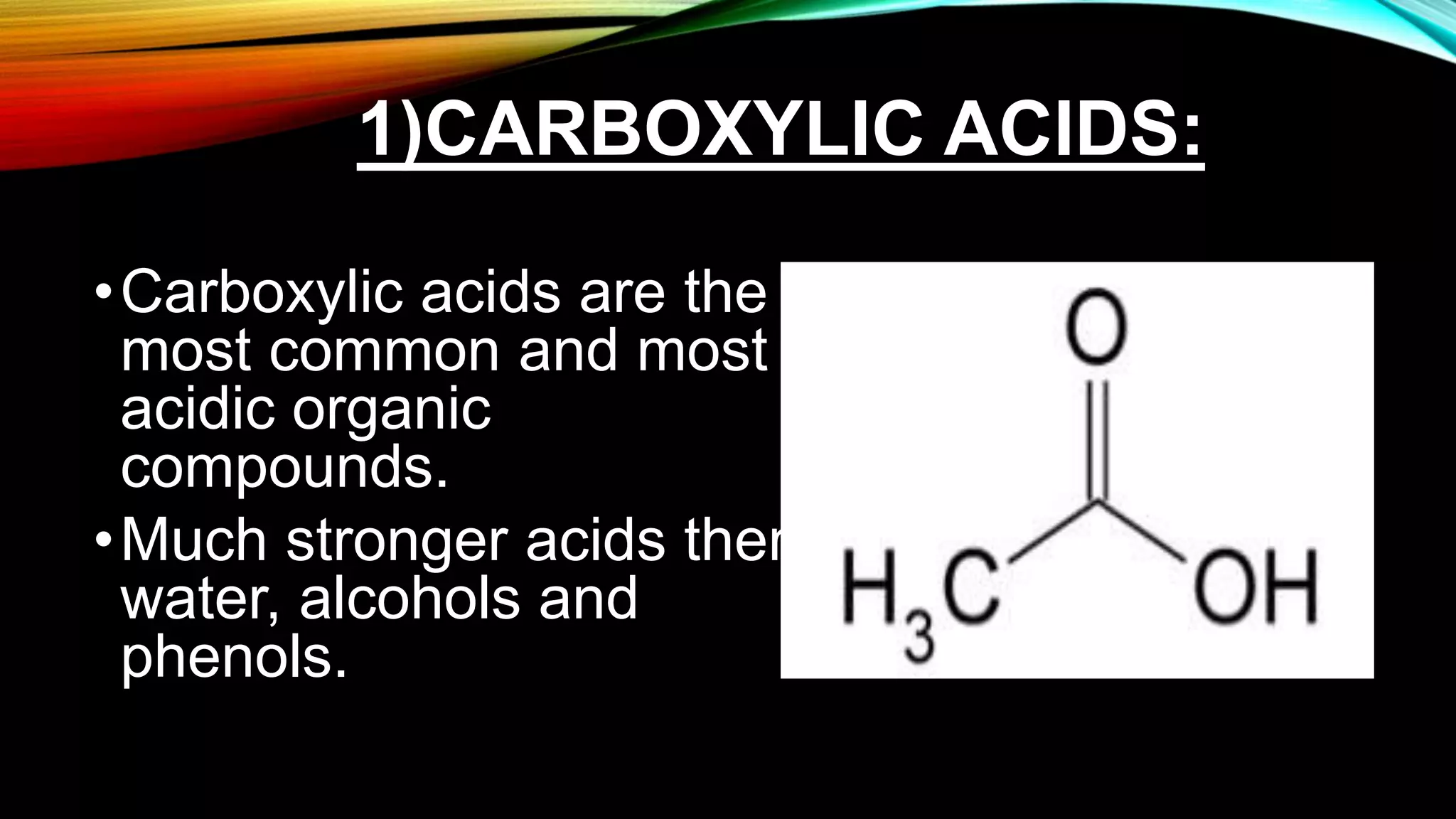
Organic acids are carbon-skeletoned compounds that partially dissociate in solution and include carboxylic acids, phenols, and alcohols. Carboxylic acids are the most common and strongest, while phenols and alcohols exhibit lower acidity. These acids have important applications in various industries, including food, pharmaceuticals, and cosmetics, and are found in numerous natural sources.